SARS-CoV-2 Nsp16 activation mechanism and a cryptic pocket with pan-coronavirus antiviral potential
- PMID: 33794150
- PMCID: PMC8007187
- DOI: 10.1016/j.bpj.2021.03.024
SARS-CoV-2 Nsp16 activation mechanism and a cryptic pocket with pan-coronavirus antiviral potential
Abstract
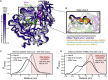
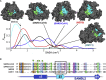
Coronaviruses have caused multiple epidemics in the past two decades, in addition to the current COVID-19 pandemic that is severely damaging global health and the economy. Coronaviruses employ between 20 and 30 proteins to carry out their viral replication cycle, including infection, immune evasion, and replication. Among these, nonstructural protein 16 (Nsp16), a 2'-O-methyltransferase, plays an essential role in immune evasion. Nsp16 achieves this by mimicking its human homolog, CMTr1, which methylates mRNA to enhance translation efficiency and distinguish self from other. Unlike human CMTr1, Nsp16 requires a binding partner, Nsp10, to activate its enzymatic activity. The requirement of this binding partner presents two questions that we investigate in this manuscript. First, how does Nsp10 activate Nsp16? Although experimentally derived structures of the active Nsp16/Nsp10 complex exist, structures of inactive, monomeric Nsp16 have yet to be solved. Therefore, it is unclear how Nsp10 activates Nsp16. Using over 1 ms of molecular dynamics simulations of both Nsp16 and its complex with Nsp10, we investigate how the presence of Nsp10 shifts Nsp16's conformational ensemble to activate it. Second, guided by this activation mechanism and Markov state models, we investigate whether Nsp16 adopts inactive structures with cryptic pockets that, if targeted with a small molecule, could inhibit Nsp16 by stabilizing its inactive state. After identifying such a pocket in SARS-CoV2 Nsp16, we show that this cryptic pocket also opens in SARS-CoV1 and MERS but not in human CMTr1. Therefore, it may be possible to develop pan-coronavirus antivirals that target this cryptic pocket.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Update of
-
SARS-CoV-2 Nsp16 activation mechanism and a cryptic pocket with pan-coronavirus antiviral potential.bioRxiv [Preprint]. 2020 Dec 10:2020.12.10.420109. doi: 10.1101/2020.12.10.420109. bioRxiv. 2020. Update in: Biophys J. 2021 Jul 20;120(14):2880-2889. doi: 10.1016/j.bpj.2021.03.024. PMID: 33330873 Free PMC article. Updated. Preprint.
Similar articles
-
SARS-CoV-2 Nsp16 activation mechanism and a cryptic pocket with pan-coronavirus antiviral potential.bioRxiv [Preprint]. 2020 Dec 10:2020.12.10.420109. doi: 10.1101/2020.12.10.420109. bioRxiv. 2020. Update in: Biophys J. 2021 Jul 20;120(14):2880-2889. doi: 10.1016/j.bpj.2021.03.024. PMID: 33330873 Free PMC article. Updated. Preprint.
-
Coronavirus nsp10/nsp16 Methyltransferase Can Be Targeted by nsp10-Derived Peptide In Vitro and In Vivo To Reduce Replication and Pathogenesis.J Virol. 2015 Aug;89(16):8416-27. doi: 10.1128/JVI.00948-15. Epub 2015 Jun 3. J Virol. 2015. PMID: 26041293 Free PMC article.
-
Structural and functional insights into the 2'-O-methyltransferase of SARS-CoV-2.Virol Sin. 2024 Aug;39(4):619-631. doi: 10.1016/j.virs.2024.07.001. Epub 2024 Jul 3. Virol Sin. 2024. PMID: 38969340 Free PMC article.
-
NSP16 2'-O-MTase in Coronavirus Pathogenesis: Possible Prevention and Treatments Strategies.Viruses. 2021 Mar 24;13(4):538. doi: 10.3390/v13040538. Viruses. 2021. PMID: 33804957 Free PMC article. Review.
-
SARS-CoV ORF1b-encoded nonstructural proteins 12-16: replicative enzymes as antiviral targets.Antiviral Res. 2014 Jan;101:122-30. doi: 10.1016/j.antiviral.2013.11.006. Epub 2013 Nov 20. Antiviral Res. 2014. PMID: 24269475 Free PMC article. Review.
Cited by
-
The Role of Coronavirus RNA-Processing Enzymes in Innate Immune Evasion.Life (Basel). 2021 Jun 17;11(6):571. doi: 10.3390/life11060571. Life (Basel). 2021. PMID: 34204549 Free PMC article. Review.
-
Antigen Delivery Platforms for Next-Generation Coronavirus Vaccines.Vaccines (Basel). 2024 Dec 31;13(1):30. doi: 10.3390/vaccines13010030. Vaccines (Basel). 2024. PMID: 39852809 Free PMC article. Review.
-
The pivotal roles of the host immune response in the fine-tuning the infection and the development of the vaccines for SARS-CoV-2.Hum Vaccin Immunother. 2021 Oct 3;17(10):3297-3309. doi: 10.1080/21645515.2021.1935172. Epub 2021 Jun 11. Hum Vaccin Immunother. 2021. PMID: 34114940 Free PMC article.
-
Antivirals for Broader Coverage against Human Coronaviruses.Viruses. 2024 Jan 20;16(1):156. doi: 10.3390/v16010156. Viruses. 2024. PMID: 38275966 Free PMC article. Review.
-
Identification and Inhibition of the Druggable Allosteric Site of SARS-CoV-2 NSP10/NSP16 Methyltransferase through Computational Approaches.Molecules. 2022 Aug 17;27(16):5241. doi: 10.3390/molecules27165241. Molecules. 2022. PMID: 36014480 Free PMC article.
References
-
- Johns Hopkins Coronavirus Resource Center . Johns Hopkins University & Medicine; 2020. COVID-19 Map. https://coronavirus.jhu.edu/map.html<span class="role">web.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

