Combining brain perturbation and neuroimaging in non-human primates
- PMID: 33794355
- PMCID: PMC11178240
- DOI: 10.1016/j.neuroimage.2021.118017
Combining brain perturbation and neuroimaging in non-human primates
Abstract
Brain perturbation studies allow detailed causal inferences of behavioral and neural processes. Because the combination of brain perturbation methods and neural measurement techniques is inherently challenging, research in humans has predominantly focused on non-invasive, indirect brain perturbations, or neurological lesion studies. Non-human primates have been indispensable as a neurobiological system that is highly similar to humans while simultaneously being more experimentally tractable, allowing visualization of the functional and structural impact of systematic brain perturbation. This review considers the state of the art in non-human primate brain perturbation with a focus on approaches that can be combined with neuroimaging. We consider both non-reversible (lesions) and reversible or temporary perturbations such as electrical, pharmacological, optical, optogenetic, chemogenetic, pathway-selective, and ultrasound based interference methods. Method-specific considerations from the research and development community are offered to facilitate research in this field and support further innovations. We conclude by identifying novel avenues for further research and innovation and by highlighting the clinical translational potential of the methods.
Keywords: Causality; Chemogenetics; Infrared; Lesion; Microstimulation; Optogenetics; Primates; Ultrasound; fMRI.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing financial interests.
Figures

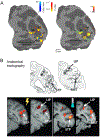
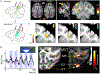

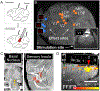
References
-
- Acker L, Pino EN, Boyden ES, Desimone R, 2016. FEF inactivation with improved optogenetic methods. PNAS 113, E7297–E7306. https://doi.org/10/f9fxqr - PMC - PubMed
-
- Acker LC, Pino EN, Boyden ES, Desimone R, 2017. Large Volume, Behaviorally-relevant Illumination for Optogenetics in Non-human Primates. J Vis Exp https://doi.org/10/gg3qpd - PMC - PubMed
-
- Adam R, Johnston K, Everling S, 2019. Recovery of contralesional saccade choice and reaction time deficits after a unilateral endothelin-1-induced lesion in the macaque caudal prefrontal cortex. Journal of Neurophysiology 122, 672–690. https://doi.org/10/gg3c57 - PubMed
-
- Adam R, Johnston K, Menon RS, Everling S, 2020. Functional reorganization during the recovery of contralesional target selection deficits after prefrontal cortex lesions in macaque monkeys. NeuroImage 207, 116339. https://doi.org/10/gg3c6r - PubMed
-
- Agustín-Pavón C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G, Asma F, Clarke H, Man M-S, Roberts AC, 2012. Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol. Psychiatry 72, 266–272. https://doi.org/10/f37gj2 - PubMed
Publication types
MeSH terms
Grants and funding
- 110157/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- G0400593/MRC_/Medical Research Council/United Kingdom
- WT092606AIA/WT_/Wellcome Trust/United Kingdom
- P51 OD011107/OD/NIH HHS/United States
- R21 AA020515/AA/NIAAA NIH HHS/United States
- ZIA EY000511/ImNIH/Intramural NIH HHS/United States
- 637638/ERC_/European Research Council/International
- MR/M023990/1/MRC_/Medical Research Council/United Kingdom
- MR/P024955/1/MRC_/Medical Research Council/United Kingdom
- R01 DC004290/DC/NIDCD NIH HHS/United States
- G0901884/MRC_/Medical Research Council/United Kingdom
- G0800329/MRC_/Medical Research Council/United Kingdom
- HHMI/Howard Hughes Medical Institute/United States
- R01 MH112142/MH/NIMH NIH HHS/United States
- ZIA MH002918/ImNIH/Intramural NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

