Emerging mechanisms and targeted therapy of ferroptosis in cancer
- PMID: 33794363
- PMCID: PMC8261167
- DOI: 10.1016/j.ymthe.2021.03.022
Emerging mechanisms and targeted therapy of ferroptosis in cancer
Abstract
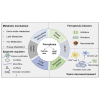
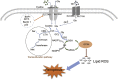
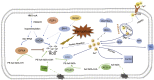
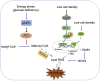
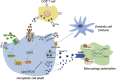
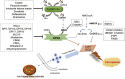
Ferroptosis is an iron- and lipid reactive oxygen species (ROS)-dependent form of programmed cell death that is distinct from other forms of regulatory cell death at the morphological, biological, and genetic levels. Emerging evidence suggests critical roles for ferroptosis in cell metabolism, the redox status, and various diseases, such as cancers, nervous system diseases, and ischemia-reperfusion injury, with ferroptosis-related proteins. Ferroptosis is inhibited in diverse cancer types and functions as a dynamic tumor suppressor in cancer development, indicating that the regulation of ferroptosis can be utilized as an interventional target for tumor treatment. Small molecules and nanomaterials that reprogram cancer cells to undergo ferroptosis are considered effective drugs for cancer therapy. Here, we systematically summarize the molecular basis of ferroptosis, the suppressive effect of ferroptosis on tumors, the effect of ferroptosis on cellular metabolism and the tumor microenvironment (TME), and ferroptosis-inducing agents for tumor therapeutics. An understanding of the latest progress in ferroptosis could provide references for proposing new potential targets for the treatment of cancers.
Keywords: cancer suppressor; cancer therapy; ferroptosis; ferroptosis-related proteins; iron; lipid ROS.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. - PubMed
-
- Eagle H. Amino acid metabolism in mammalian cell cultures. Science. 1959;130:432–437. - PubMed
-
- Darnell J.E., Jr., Eagle H. Glucose and glutamine in poliovirus production by HeLa cells. Virology. 1958;6:556–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

