Correlation between loss of Smad4 and clinical parameters of non-small cell lung cancer: an observational cohort study
- PMID: 33794845
- PMCID: PMC8017835
- DOI: 10.1186/s12890-021-01480-z
Correlation between loss of Smad4 and clinical parameters of non-small cell lung cancer: an observational cohort study
Abstract
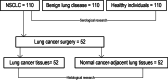
Background: SMAD4 has been found to be inactivated to varying degrees in many types of cancer; the purpose of this study was to investigate the correlation between SMAD4 expression in non-small cell lung cancer (NSCLC) and clinical pathological parameters.
Methods: The serum concentration of SMAD4 was measured by enzyme-linked immunosorbent assay and its histological expression was quantified by immunohistochemistry.
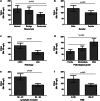
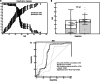
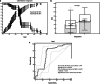
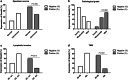
Results: The serum concentration of Smad4 in patients with NSCLC was lower than that in benign lung disease patients and healthy individuals (P < 0.001) and its concentration was related to the histological classification, pathological differentiation, lymphatic metastasis and clinical stage of NSCLC. The sensitivity and specificity of serum Smad4 were 91.56% and 61.56% for screening NSCLC from healthy individuals and 84.55% and 60.36% for screening NSCLC from patients with benign lung disease. Logistic regression analysis showed that the degree of cell differentiation (P < 0.001), lymph node metastasis (P < 0.001) and clinical stage of NSCLC (P = 0.007) affected the expression of Smad4, and had a strong correlation with the expression of Smad4. The expression of Smad4 in NSCLC tissues was lower than that in normal lung tissues (P = 0.009) and its expression was related to the degree of tissue differentiation, lymph node metastasis and clinical stage (P < 0.05).
Conclusions: The downregulation or deletion of Smad4 is related to the malignant biological behavior of NSCLC and serum Smad4 could be considered as a potential molecular indicator for diagnosis and evaluation of NSCLC.
Keywords: Diagnosis; Evaluation; NSCLC; Non-small cell lung cancer; Smad4.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Micke P, Mattsson JS, Djureinovic D, Nodin B, Jirström K, Tran L, Jönsson P, Planck M, Botling J, Brunnström H. The impact of the fourth edition of the WHO classification of lung tumours on histological classification of resected pulmonary NSCCs. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2016;11(6):862–872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

