CREB-dependent LPA-induced signaling initiates a pro-fibrotic feedback loop between small airway basal cells and fibroblasts
- PMID: 33794877
- PMCID: PMC8015171
- DOI: 10.1186/s12931-021-01677-0
CREB-dependent LPA-induced signaling initiates a pro-fibrotic feedback loop between small airway basal cells and fibroblasts
Abstract
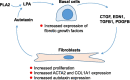
Background: Lysophosphatidic acid (LPA), generated extracellularly by the action of autotaxin and phospholipase A2, functions through LPA receptors (LPARs) or sphingosine-1-phosphate receptors (S1PRs) to induce pro-fibrotic signaling in the lower respiratory tract of patients with idiopathic pulmonary fibrosis (IPF). We hypothesized that LPA induces changes in small airway epithelial (SAE) basal cells (BC) that create cross-talk between the BC and normal human lung fibroblasts (NHLF), enhancing myofibroblast formation.
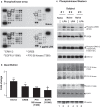
Methods: To assess LPA-induced signaling, BC were treated with LPA for 2.5 min and cell lysates were analyzed by phosphokinase array and Western blot. To assess transcriptional changes, BC were treated with LPA for 3 h and harvested for collection and analysis of RNA by quantitative polymerase chain reaction (qPCR). To assess signaling protein production and function, BC were washed thoroughly after LPA treatment and incubated for 24 h before collection for protein analysis by ELISA or functional analysis by transfer of conditioned medium to NHLF cultures. Transcription, protein production, and proliferation of NHLF were assessed.
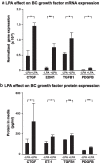
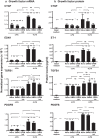
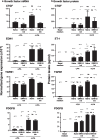
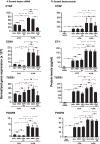
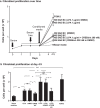
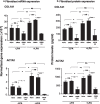
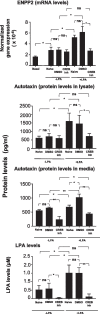
Results: LPA treatment induced signaling by cAMP response element-binding protein (CREB), extracellular signal-related kinases 1 and 2 (Erk1/2), and epithelial growth factor receptor (EGFR) resulting in elevated expression of connective tissue growth factor (CTGF), endothelin-1 (EDN1/ET-1 protein), and platelet derived growth factor B (PDGFB) at the mRNA and protein levels. The conditioned medium from LPA-treated BC induced NHLF proliferation and increased NHLF expression of collagen I (COL1A1), smooth muscle actin (ACTA2), and autotaxin (ENPP2) at the mRNA and protein levels. Increased autotaxin secretion from NHLF correlated with increased LPA in the NHLF culture medium. Inhibition of CREB signaling blocked LPA-induced changes in BC transcription and translation as well as the pro-fibrotic effects of the conditioned medium on NHLF.
Conclusion: Inhibition of CREB signaling may represent a novel target for alleviating the LPA-induced pro-fibrotic feedback loop between SAE BC and NHLF.
Keywords: ACTA2; Airway basal cell; Autotaxin; COL1A1; CREB; Fibroblast; Idiopathic pulmonary fibrosis; Intracellular signaling; Lung; Lysophosphatidic acid.
Conflict of interest statement
The authors declare that they have no competing interests.
The authors have declared that no conflict of interest exists.
Figures
References
-
- Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, et al. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2002;166:236–246. doi: 10.1164/rccm.2201069. - DOI - PubMed
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

