Single nucleotide polymorphisms associated with methotrexate-induced nausea in juvenile idiopathic arthritis
- PMID: 33794950
- PMCID: PMC8017639
- DOI: 10.1186/s12969-021-00539-9
Single nucleotide polymorphisms associated with methotrexate-induced nausea in juvenile idiopathic arthritis
Abstract
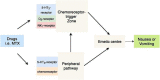
Background: Context: Methotrexate (MTX) is a cornerstone in the treatment of juvenile idiopathic arthritis (JIA). MTX treatment is commonly associated with nausea. Large inter-individual variation exists in the level of MTX-induced nausea, possibly due to genetic factors.
Purpose: To investigate whether MTX-induced nausea was associated with single nucleotide polymorphisms (SNPs) in genes encoding MTX-transporter proteins, a MTX metabolizing enzyme and a nausea receptor.
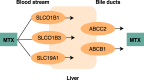
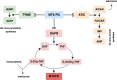
Findings: Methods: Children aged ≥9 years treated with MTX for JIA were eligible. MTX-induced nausea was registered by the children's completion of a nausea diary (min. 7 days) and the parents' completion of the MTX intolerance severity score (MISS). The selected SNPs were: SLCO1B1 (rs4149056; rs4149081), SLCO1B3 (rs2117032), SLC19A1 (rs1051266), ABCC2 (rs2273697; rs3740066; rs717620), ABCB1 (rs2032582; rs1045642), MTHFR (rs1801131, rs1801133), HTR3A (rs1062613; rs1985242; rs1176713) and HTR3B (rs1176744).
Result: Enrolled were 121 JIA patients (82 girls: 39 boys) with a median age of 13.3 years (IQR: 11.3-15.1). The median MTX dose was 9.7 mg/m2/week (IQR: 9.0-10.9). The median MTX treatment duration prior to enrolment was 340 days (IQR: 142-766). The SNP analysis was available for 119 patients. MTX intolerance was associated with the genotype distribution of rs1801133 (MTHFR) (p = 0.02). There was no additive effect of the minor alleles for any of the selected SNPs, nor any significant haplotype associations.
Conclusion: Summary: MTX-induced nausea may be influenced by genetic polymorphisms in a MTX metabolizing enzyme (rs1801133; MTHFR).
Implications: Further analyses involving inclusion of larger cohorts are needed to understand the impact of SNPs on MTX-induced nausea in JIA.
Keywords: JIA; Juvenile idiopathic arthritis; MTX; Methotrexate; SNPs; Single nucleotide polymorphisms.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.a.-U.S.S.R. double-blind, placebo-controlled trial. The pediatric rheumatology collaborative study group and the cooperative Children's study group. N Engl J Med. 1992;326(16):1043–9. 10.1056/NEJM199204163261602. - PubMed
-
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63(4):465–482. doi: 10.1002/acr.20460. - DOI - PMC - PubMed
-
- Ruperto N, Murray KJ, Gerloni V, Wulffraat N, de Oliveira SK, Falcini F, et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50(7):2191–2201. doi: 10.1002/art.20288. - DOI - PubMed
-
- Brunner HI, Johnson AL, Barron AC, Passo MH, Griffin TA, Graham TB, et al. Gastrointestinal symptoms and their association with health-related quality of life of children with juvenile rheumatoid arthritis: validation of a gastrointestinal symptom questionnaire. J Clin Rheumatol. 2005;11(4):194–204. 10.1097/01.rhu.0000173616.81928.44. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

