Oral administration of repurposed drug targeting Cyp46A1 increases survival times of prion infected mice
- PMID: 33795005
- PMCID: PMC8017635
- DOI: 10.1186/s40478-021-01162-1
Oral administration of repurposed drug targeting Cyp46A1 increases survival times of prion infected mice
Abstract
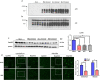
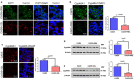
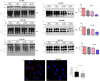
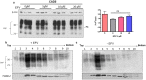
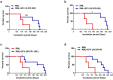
Prion diseases are fatal, infectious, and incurable neurodegenerative disorders caused by misfolding of the cellular prion protein (PrPC) into the infectious isoform (PrPSc). In humans, there are sporadic, genetic and infectious etiologies, with sporadic Creutzfeldt-Jakob disease (sCJD) being the most common form. Currently, no treatment is available for prion diseases. Cellular cholesterol is known to impact prion conversion, which in turn results in an accumulation of cholesterol in prion-infected neurons. The major elimination of brain cholesterol is achieved by the brain specific enzyme, cholesterol 24-hydroxylase (CYP46A1). Cyp46A1 converts cholesterol into 24(S)-hydroxycholesterol, a membrane-permeable molecule that exits the brain. We have demonstrated for the first time that Cyp46A1 levels are reduced in the brains of prion-infected mice at advanced disease stage, in prion-infected neuronal cells and in post-mortem brains of sCJD patients. We have employed the Cyp46A1 activator efavirenz (EFV) for treatment of prion-infected neuronal cells and mice. EFV is an FDA approved anti-HIV medication effectively crossing the blood brain barrier and has been used for decades to chronically treat HIV patients. EFV significantly mitigated PrPSc propagation in prion-infected cells while preserving physiological PrPC and lipid raft integrity. Notably, oral administration of EFV treatment chronically at very low dosage starting weeks to months after intracerebral prion inoculation of mice significantly prolonged the lifespan of animals. In summary, our results suggest that Cyp46A1 as a novel therapeutic target and that its activation through repurposing the anti-retroviral medication EFV might be valuable treatment approach for prion diseases.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

