Neuropathological and behavioral characterization of aged Grn R493X progranulin-deficient frontotemporal dementia knockin mice
- PMID: 33795008
- PMCID: PMC8017751
- DOI: 10.1186/s40478-021-01158-x
Neuropathological and behavioral characterization of aged Grn R493X progranulin-deficient frontotemporal dementia knockin mice
Abstract
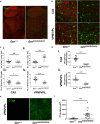
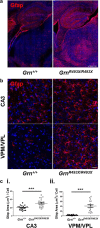
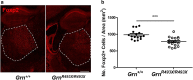
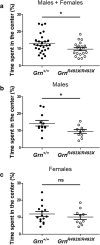
Frontotemporal lobar degeneration (FTLD) causes a spectrum of clinical presentations of frontotemporal dementia (FTD), including progressive changes in behavior, personality, executive function, and language. Up to 20% of familial FTLD cases are caused by progranulin (GRN) haploinsufficiency (FTD-GRN), with one of the most common causal variant being a nonsense mutation at arginine 493 (R493X). Recently, a genetic knockin FTD-GRN mouse model was generated bearing this GrnR493X mutation, at the analogous arginine in murine Grn. Aged, homozygous GrnR493X mice (GrnR493X/R493X) have been shown to phenotypically replicate several neuropathological hallmarks previously demonstrated in Grn null mice. We conducted a comprehensive neuropathological and behavioral assessment of 18 month old GrnR493X/R493X mice, observing a striking lysosomal dysfunction and thalamic neurodegeneration not previously described in this model, as well as a male-specific increase in generalized anxiety. These findings provide additional phenotypic markers of pathogenesis in aged GrnR493X/R493X mice that will contribute to better defining mechanisms underlying FTD-GRN, and offer relevant outcome measures for preclinical efficacy testing of novel therapeutics that target nonsense mutations leading to this devastating disease.
Keywords: Astrogliosis; Frontotemporal dementia; Lysosomal dysfunction; Microgliosis; Mouse model; Neurodegeneration; Open field; Progranulin; R493X; TDP-43.
Conflict of interest statement
H.B.N. has received royalties as an independent consultant for Biogen and Roche Canada.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

