Cardiovascular Risk in Patients With Psoriasis: JACC Review Topic of the Week
- PMID: 33795041
- PMCID: PMC8168628
- DOI: 10.1016/j.jacc.2021.02.009
Cardiovascular Risk in Patients With Psoriasis: JACC Review Topic of the Week
Abstract
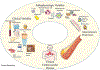
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of the U.S. population. The immune response in psoriasis includes enhanced activation of T cells and myeloid cells, platelet activation, and up-regulation of interferons, tumor necrosis factor-α, and interleukins (ILs) IL-23, IL-17, and IL-6, which are linked to vascular inflammation and atherosclerosis development. Patients with psoriasis are up to 50% more likely to develop cardiovascular disease (CV) disease, and this CV risk increases with skin severity. Major society guidelines now advocate incorporating a psoriasis diagnosis into CV risk prediction and prevention strategies. Although registry data suggest treatment targeting psoriasis skin disease reduces vascular inflammation and coronary plaque burden, and may reduce CV risk, randomized placebo-controlled trials are inconclusive to date. Further studies are required to define traditional CV risk factor goals, the optimal role of lipid-lowering and antiplatelet therapy, and targeted psoriasis therapies on CV risk.
Keywords: cardiovascular disease; cardiovascular risk; inflammation; psoriasis.
Copyright © 2021 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Financial support was provided, in part, by an American Heart Association Career Development Grant 18CDA34080540 and National Psoriasis Foundation Bridge Grant, (to Dr. Garshick). Dr. Ward was supported, in part, by National Institutes of Health grants P50AR070590, R01AR063437, and R01AR073196. Dr. Krueger has received grants from Novartis, Pfizer, Amgen, Lilly, Boehringer, Innovaderm, Bristol Myers Squibb, Janssen, Abbvie, Paraxel, Leo Pharma, Vitae, Akros, Regeneron, Allergan, Novan, Biogen MA, Sienna, UCB, Celgene, Botanix, Incyte, Avillion, and Exicure; has received personal fees from Novartis, Pfizer, Amgen, Lilly, Boehringer, BiogenIdec, Abbvie, Leo Pharma, Escalier, Valeant, Aurigne, Allergan, Asana, UCB, Sienna, Celgene, Nimbus, Menlo, Aristea, Sanofi, Sun Pharma, Almirall, Arena, and Bristol Myers Squibb. Dr. Berger was supported, in part, by National Institutes of Health grants R01HL139909, R01HL114978, and R35HL144993.
Figures



References
-
- Benjamin EJ, Muntner P, Alonso A et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. - PubMed
-
- Stern R Discordance of individual risk estimates. J Am Coll Cardiol 2010;56:743; author reply 743–4. - PubMed
-
- Greb JE, Goldminz AM, Elder JT et al. Psoriasis. Nat Rev Dis Primers 2016;2:16082. - PubMed
-
- McDonald CJ, Calabresi P. Psoriasis and occlusive vascular disease. Br J Dermatol 1978;99:469–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

