Randomised clinical trial for the cost-utility evaluation of two strategies of perineal reconstruction after abdominoperineal resection in the context of anorectal carcinoma: biological mesh repair versus primary perineal wound closure, study protocol for the GRECCAR 9 Study
- PMID: 33795299
- PMCID: PMC8021762
- DOI: 10.1136/bmjopen-2020-043333
Randomised clinical trial for the cost-utility evaluation of two strategies of perineal reconstruction after abdominoperineal resection in the context of anorectal carcinoma: biological mesh repair versus primary perineal wound closure, study protocol for the GRECCAR 9 Study
Abstract
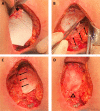
Introduction: Abdominoperineal resections performed for anorectal tumours leave a large pelvic and perineal defect causing a high rate of morbidity of the perineal wound (40%-60%). Biological meshes offer possibilities for new standards of perineal wound reconstruction. Perineal fillings with biological mesh are expected to increase quality of life by reducing perineal morbidity.
Methods and analysis: This is a multicentre, randomised and single-blinded study with a blinded endpoint evaluation, the experimental arm of which uses a biological mesh and the control arm of which is defined by the primary closure after abdominoperineal resection for cancer. Patients eligible for inclusion are patients with a proven history of rectal adenocarcinoma and anal canal epidermoid carcinoma for whom abdominoperineal resection was indicated after a multidisciplinary team discussion. All patients must have social security insurance or equivalent social protection. The main objective is to assess the incremental cost-utility ratio (ICUR) of two strategies of perineal closure after an abdominoperineal resection performed for anorectal cancer treatment: perineal filling with biological mesh versus primary perineal closure (70 patient in each arm). The secondary objectives focus on quality of life and morbidity data during a 1-year follow-up. Deterministic and probabilistic sensitivity analyses will be performed in order to estimate the uncertainty surrounding the ICUR. CIs will be constructed using the non-parametric bootstrap approach. A cost-effectiveness acceptability curve will be built so as to estimate the probability of efficiency of the biological meshes given a collective willingness-to-pay threshold.
Ethics and dissemination: The study was approved by the Regional Ethical Review Board of 'Nord Ouest 1' (protocol reference number: 20.05.14.60714; national number: 2020-A01169-30).The results will be disseminated through conventional scientific channels.
Trial registration number: ClinicalTrials.gov Registry (NCT02841293).
Keywords: colorectal surgery; health economics; oncology; wound management.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Musters GD, Buskens CJ, Bemelman WA. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2014;57:1129–39. - PubMed
-
- Devulapalli C, ATJ W, Dibiagio JR. Primary versus flap closure of perineal defects following oncologic resection: a systematic review and meta-analysis. Plast Reconstr Surg 2016;137:1602–13. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
