SARS-CoV-2 population-based seroprevalence studies in Europe: a scoping review
- PMID: 33795310
- PMCID: PMC8021754
- DOI: 10.1136/bmjopen-2020-045425
SARS-CoV-2 population-based seroprevalence studies in Europe: a scoping review
Abstract
Objectives: We aimed to review SARS-CoV-2 seroprevalence studies conducted in Europe to understand how they may be used to inform ongoing control strategies for COVID-19.
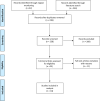
Design: Scoping review of peer-reviewed publications and manuscripts on preprint servers from January 2020 to 15 September 2020.
Primary measure: Seroprevalence estimate (and lower and upper CIs). For studies conducted across a country or territory, we used the seroprevalence estimate and the upper and lower CIs and compared them to the total number of reported infections to calculate the ratio of reported to expected infections.
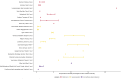
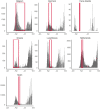
Results: We identified 23 population-based seroprevalence studies conducted in Europe. Among 12 general population studies, seroprevalence ranged from 0.42% among residual clinical samples in Greece to 13.6% in an area of high transmission in Gangelt, Germany. Of the eight studies in blood donors, seroprevalence ranged from 0.91% in North-Western Germany to 23.3% in a high-transmission area in Lombardy region, Italy. In three studies which recruited individuals through employment, seroprevalence ranged from 0.5% among factory workers in Frankfurt, Germany, to 10.2% among university employees in Milan, Italy. In comparison to nationally reported cases, the extent of infection, as derived from these seroprevalence estimates, is manyfold higher and largely heterogeneous.
Conclusion: Exposure to the virus in Europe has not reached a level of infection that would prevent further circulation of the virus. Effective vaccine candidates are urgently required to deliver the level of immunity in the population.
Keywords: epidemiology; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Bobrovitz N, Arora RK, Yan T. Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. MedRxiv 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
