Inhibition of Human Uracil DNA Glycosylase Sensitizes a Large Fraction of Colorectal Cancer Cells to 5-Fluorodeoxyuridine and Raltitrexed but Not Fluorouracil
- PMID: 33795350
- PMCID: PMC11033954
- DOI: 10.1124/molpharm.120.000191
Inhibition of Human Uracil DNA Glycosylase Sensitizes a Large Fraction of Colorectal Cancer Cells to 5-Fluorodeoxyuridine and Raltitrexed but Not Fluorouracil
Abstract
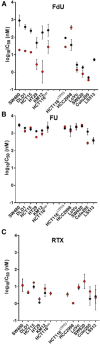
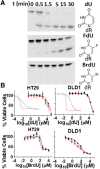
Previous short-hairpin RNA knockdown studies have established that depletion of human uracil DNA glycosylase (hUNG) sensitizes some cell lines to 5-fluorodeoxyuridine (FdU). Here, we selectively inhibit the catalytic activity of hUNG by lentiviral transduction of uracil DNA glycosylase inhibitor protein into a large panel of cancer cell lines under control of a doxycycline-inducible promoter. This induced inhibition strategy better assesses the therapeutic potential of small-molecule targeting of hUNG. In total, 6 of 11 colorectal lines showed 6- to 70-fold increases in FdU potency upon hUNG inhibition ("responsive"). This hUNG-dependent response was not observed with fluorouracil (FU), indicating that FU does not operate through the same DNA repair mechanism as FdU in vitro. Potency of the thymidylate synthase inhibitor raltitrexed (RTX), which elevates deoxyuridine triphosphate levels, was only incrementally enhanced upon hUNG inhibition (<40%), suggesting that responsiveness is associated with incorporation and persistence of FdU in DNA rather than deoxyuridine. The importance of FU/A and FU/G lesions in the toxicity of FdU is supported by the observation that dT supplementation completely rescued the toxic effects of U/A lesions resulting from RTX, but dT only increased the IC50 for FdU, which forms both FU/A and FU/G mismatches. Contrary to previous reports, cellular responsiveness to hUNG inhibition did not correlate with p53 status or thymine DNA glycosylase expression. A model is suggested in which the persistence of FU/A and FU/G base pairs in the absence of hUNG activity elicits an apoptotic DNA damage response in both responsive and nonresponsive colorectal lines. SIGNIFICANCE STATEMENT: The pyrimidine base 5-fluorouracil is a mainstay chemotherapeutic for treatment of advanced colorectal cancer. Here, this study shows that its deoxynucleoside form, 5-fluorodeoxyuridine (FdU), operates by a distinct DNA incorporation mechanism that is strongly potentiated by inhibition of the DNA repair enzyme human uracil DNA glycosylase. The hUNG-dependent mechanism was present in over 50% of colorectal cell lines tested, suggesting that a significant fraction of human cancers may be sensitized to FdU in the presence of a small-molecule hUNG inhibitor.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors report no conflicts of interest.
Figures







References
-
- Benson AB III, Venook AP, Cederquist L, Chan E, Chen Y-J, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, et al. (2017) Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:370–398. - PubMed
-
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB (2005) Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 123:437–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

