Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria
- PMID: 33795655
- PMCID: PMC8016968
- DOI: 10.1038/s41419-021-03643-6
Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria
Abstract
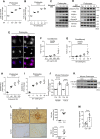
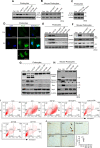
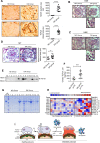
Glomerular podocytes are integral members of the glomerular filtration barrier in the kidney and are crucial for glomerular permselectivity. These highly differentiated cells are vulnerable to an array of noxious stimuli that prevail in several glomerular diseases. Elevated circulating growth hormone (GH) levels are associated with podocyte injury and proteinuria in diabetes. However, the precise mechanism(s) by which excess GH elicits podocytopathy remains to be elucidated. Previous studies have shown that podocytes express GH receptor (GHR) and induce Notch signaling when exposed to GH. In the present study, we demonstrated that GH induces TGF-β1 signaling and provokes cell cycle reentry of otherwise quiescent podocytes. Though differentiated podocytes reenter the cell cycle in response to GH and TGF-β1, they cannot accomplish cytokinesis, despite karyokinesis. Owing to this aberrant cell cycle event, GH- or TGF-β1-treated cells remain binucleated and undergo mitotic catastrophe. Importantly, inhibition of JAK2, TGFBR1 (TGF-β receptor 1), or Notch prevented cell cycle reentry of podocytes and protected them from mitotic catastrophe associated with cell death. Inhibition of Notch activation prevents GH-dependent podocyte injury and proteinuria. Similarly, attenuation of GHR expression abated Notch activation in podocytes. Kidney biopsy sections from patients with diabetic nephropathy (DN) show activation of Notch signaling and binucleated podocytes. These data indicate that excess GH induced TGF-β1-dependent Notch1 signaling contributes to the mitotic catastrophe of podocytes. This study highlights the role of aberrant GH signaling in podocytopathy and the potential application of TGF-β1 or Notch inhibitors, as a therapeutic agent for DN.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy.J Biol Chem. 2019 Nov 1;294(44):16109-16122. doi: 10.1074/jbc.RA119.008966. Epub 2019 Sep 11. J Biol Chem. 2019. PMID: 31511328 Free PMC article.
-
Growth hormone induces transforming growth factor-β1 in podocytes: Implications in podocytopathy and proteinuria.Biochim Biophys Acta Mol Cell Res. 2023 Feb;1870(2):119391. doi: 10.1016/j.bbamcr.2022.119391. Epub 2022 Nov 15. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36400249
-
Growth Hormone Induces Transforming Growth Factor-Beta-Induced Protein in Podocytes: Implications for Podocyte Depletion and Proteinuria.J Cell Biochem. 2015 Sep;116(9):1947-56. doi: 10.1002/jcb.25150. J Cell Biochem. 2015. PMID: 25740786
-
Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy.Curr Diabetes Rev. 2008 Feb;4(1):39-45. doi: 10.2174/157339908783502370. Curr Diabetes Rev. 2008. PMID: 18220694 Review.
-
The glomerular podocyte as a target of growth hormone action: implications for the pathogenesis of diabetic nephropathy.Curr Diabetes Rev. 2011 Jan;7(1):50-5. doi: 10.2174/157339911794273900. Curr Diabetes Rev. 2011. PMID: 21067510 Free PMC article. Review.
Cited by
-
Editorial: Endocrine abnormalities and renal complications.Front Endocrinol (Lausanne). 2023 Aug 21;14:1274669. doi: 10.3389/fendo.2023.1274669. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37670892 Free PMC article. No abstract available.
-
HOXA11-OS participates in lupus nephritis by targeting miR-124-3p mediating Cyr61 to regulate podocyte autophagy.Mol Med. 2022 Nov 22;28(1):138. doi: 10.1186/s10020-022-00570-w. Mol Med. 2022. PMID: 36418932 Free PMC article.
-
Identification and validation of immune-related biomarkers and potential regulators and therapeutic targets for diabetic kidney disease.BMC Med Genomics. 2023 May 1;16(1):90. doi: 10.1186/s12920-023-01519-6. BMC Med Genomics. 2023. PMID: 37127580 Free PMC article.
-
Cell cycle disorders in podocytes: an emerging and increasingly recognized phenomenon.Cell Death Discov. 2025 Apr 17;11(1):182. doi: 10.1038/s41420-025-02486-w. Cell Death Discov. 2025. PMID: 40246828 Free PMC article. Review.
-
Insight into the Molecular Mechanism of Diabetic Kidney Disease and the Role of Metformin in Its Pathogenesis.Int J Mol Sci. 2023 Aug 22;24(17):13038. doi: 10.3390/ijms241713038. Int J Mol Sci. 2023. PMID: 37685845 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

