ZIP8 exacerbates collagen-induced arthritis by increasing pathogenic T cell responses
- PMID: 33795795
- PMCID: PMC8102558
- DOI: 10.1038/s12276-021-00591-1
ZIP8 exacerbates collagen-induced arthritis by increasing pathogenic T cell responses
Erratum in
-
Author Correction: ZIP8 exacerbates collagen-induced arthritis by increasing pathogenic T cell responses.Exp Mol Med. 2025 Jul;57(7):1610-1611. doi: 10.1038/s12276-025-01469-2. Exp Mol Med. 2025. PMID: 40664883 Free PMC article. No abstract available.
Abstract
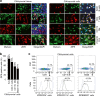
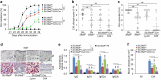
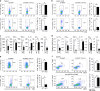
Zinc is a trace element that is essential for immune responses. Therefore, changes in cellular zinc levels in specific immune cells may influence inflammatory autoimmune diseases, such as rheumatoid arthritis (RA). However, the regulation of zinc mobilization in immune cells and its role in the pathogenesis of RA are not fully understood. Thus, we investigated the roles of zinc transporters in RA pathogenesis. We demonstrated that ZIP8 was specifically upregulated in CD4+ T cells that infiltrated the inflamed joint and that ZIP8 deficiency in CD4+ T cells abrogated collagen-induced arthritis. ZIP8 deficiency dramatically affected zinc influx in effector T cells and profoundly reduced T cell receptor (TCR)-mediated signaling, including NF-κB and MAPK signaling, which are pathways that are involved in T helper (Th) 17 cell differentiation. Taken together, our findings suggest that ZIP8 depletion in CD4+ T cells attenuates TCR signaling due to insufficient cellular zinc, thereby reducing the function of effector CD4+ T cells, including Th17 cells. Our results also suggest that targeting ZIP8 may be a useful strategy to inhibit RA development and pathogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med.365, 2205–2219 (2011). - PubMed
-
- Karami, J., Aslani, S., Jamshidi, A., Garshasbi, M. & Mahmoudi, M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene702, 8–16 (2019). - PubMed
-
- Okada, Y., Eyre, S., Suzuki, A., Kochi, Y. & Yamamoto, K. Genetics of rheumatoid arthritis: 2018 status. Ann. Rheum. Dis.78, 446–453 (2019). - PubMed
-
- McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol.7, 429–442 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

