Spike Generators and Cell Signaling in the Human Auditory Nerve: An Ultrastructural, Super-Resolution, and Gene Hybridization Study
- PMID: 33796009
- PMCID: PMC8008129
- DOI: 10.3389/fncel.2021.642211
Spike Generators and Cell Signaling in the Human Auditory Nerve: An Ultrastructural, Super-Resolution, and Gene Hybridization Study
Abstract
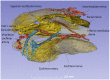
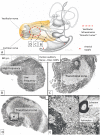
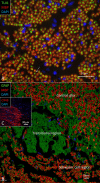
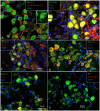
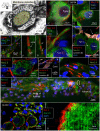
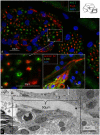
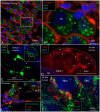
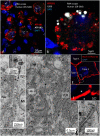
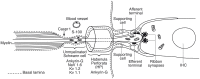
Background: The human auditory nerve contains 30,000 nerve fibers (NFs) that relay complex speech information to the brain with spectacular acuity. How speech is coded and influenced by various conditions is not known. It is also uncertain whether human nerve signaling involves exclusive proteins and gene manifestations compared with that of other species. Such information is difficult to determine due to the vulnerable, "esoteric," and encapsulated human ear surrounded by the hardest bone in the body. We collected human inner ear material for nanoscale visualization combining transmission electron microscopy (TEM), super-resolution structured illumination microscopy (SR-SIM), and RNA-scope analysis for the first time. Our aim was to gain information about the molecular instruments in human auditory nerve processing and deviations, and ways to perform electric modeling of prosthetic devices. Material and Methods: Human tissue was collected during trans-cochlear procedures to remove petro-clival meningioma after ethical permission. Cochlear neurons were processed for electron microscopy, confocal microscopy (CM), SR-SIM, and high-sensitive in situ hybridization for labeling single mRNA transcripts to detect ion channel and transporter proteins associated with nerve signal initiation and conductance. Results: Transport proteins and RNA transcripts were localized at the subcellular level. Hemi-nodal proteins were identified beneath the inner hair cells (IHCs). Voltage-gated ion channels (VGICs) were expressed in the spiral ganglion (SG) and axonal initial segments (AISs). Nodes of Ranvier (NR) expressed Nav1.6 proteins, and encoding genes critical for inter-cellular coupling were disclosed. Discussion: Our results suggest that initial spike generators are located beneath the IHCs in humans. The first NRs appear at different places. Additional spike generators and transcellular communication may boost, sharpen, and synchronize afferent signals by cell clusters at different frequency bands. These instruments may be essential for the filtering of complex sounds and may be challenged by various pathological conditions.
Keywords: auditory nerve; gene expression; human; spike generation; structured illumination microscopy.
Copyright © 2021 Liu, Luque, Li, Schrott-Fischer, Glueckert, Tylstedt, Rajan, Ladak, Agrawal and Rask-Andersen.
Conflict of interest statement
MED-EL Medical Electronics, R&D, GmbH, and Innsbruck, Austria provided salary support for one research group member (WL) in accordance with the contract agreement with Uppsala University, Sweden during 2018. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Arnold W., Wang J. B., Linnenkohl S. (1980). [Anatomical observations in the spiral ganglion of human newborns (author's transl)]. Arch. Otorhinolaryngol. 228, 69–84. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

