Positive Effect of Andrographolide Induced Autophagy on Random-Pattern Skin Flaps Survival
- PMID: 33796027
- PMCID: PMC8008123
- DOI: 10.3389/fphar.2021.653035
Positive Effect of Andrographolide Induced Autophagy on Random-Pattern Skin Flaps Survival
Abstract
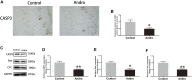
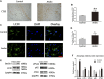
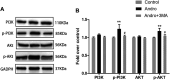
Random-pattern skin flap replantation is generally used in the reconstruction of surgical tissues and covering a series of skin flap defects. However, ischemia often occurs at the flap distal parts, which lead to flap necrosis. Previous studies have shown that andrographolide (Andro) protects against ischemic cardiovascular diseases, but little is known about the effect of Andro on flap viability. Thus, our study aimed to building a model of random-pattern skin flap to understand the mechanism of Andro-induced effects on flap survival. In this study, fifty-four mice were randomly categorized into the control, Andro group, and the Andro+3-methyladenine group. The skin flap samples were obtained on postoperative day 7. Subsequently, the tissue samples were underwent a series of evaluations such as changes in the appearance of flap tissue, the intensity of blood flow, and neovascularization density of skin flap. In our study, the results revealed that Andro enhanced the viability of random skin flaps by enhancing angiogenesis, inhibiting apoptosis, and reducing oxidative stress. Furthermore, our results have also demonstrated that the administration of Andro caused an elevation in the autophagy, and these remarkable impact of Andro were reversed by 3-methyladenine (3-MA), the most common autophagy inhibitor. Together, our data proves novel evidence that Andro is a potent modulator of autophagy capable of significantly increasing random-pattern skin flap survival.
Keywords: PI3K/Akt signaling pathway; andrographolide; angiogenesis; apoptosis; autophagy; oxidative stress; random-pattern flap.
Copyright © 2021 Jiang, Jin, Lou, Li, Wu, Cheng, Dong, Chen and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Chehelcheraghi F., Eimani H., Homayoonsadraie S., Torkaman G., Amini A., Alavi Majd H., et al. (2016). Effects of acellular amniotic membrane matrix and bone marrow-derived mesenchymal stem cells in improving random skin flap survival in rats. Iran Red Crescent Med. J. 18 (6), e25588. 10.5812/ircmj.25588 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

