Activation of Neutrophil Granulocytes by Platelet-Activating Factor Is Impaired During Experimental Sepsis
- PMID: 33796110
- PMCID: PMC8007865
- DOI: 10.3389/fimmu.2021.642867
Activation of Neutrophil Granulocytes by Platelet-Activating Factor Is Impaired During Experimental Sepsis
Abstract
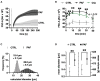
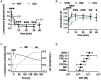
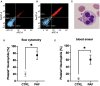
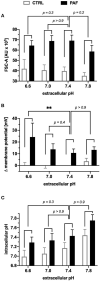
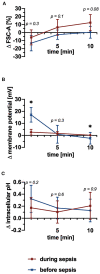
Platelet-activating factor (PAF) is an important mediator of the systemic inflammatory response. In the case of sepsis, proper activation and function of neutrophils as the first line of cellular defense are based on a well-balanced physiological response. However, little is known about the role of PAF in cellular changes of neutrophils during sepsis. Therefore, this study investigates the reaction patterns of neutrophils induced by PAF with a focus on membrane potential (MP), intracellular pH, and cellular swelling under physiological and pathophysiological conditions and hypothesizes that the PAF-mediated response of granulocytes is altered during sepsis. The cellular response of granulocytes including MP, intracellular pH, cellular swelling, and other activation markers were analyzed by multiparametric flow cytometry. In addition, the chemotactic activity and the formation of platelet-neutrophil complexes after exposure to PAF were investigated. The changes of the (electro-)physiological response features were translationally verified in a human ex vivo whole blood model of endotoxemia as well as during polymicrobial porcine sepsis. In neutrophils from healthy human donors, PAF elicited a rapid depolarization, an intracellular alkalization, and an increase in cell size in a time- and dose-dependent manner. Mechanistically, the alkalization was dependent on sodium-proton exchanger 1 (NHE1) activity, while the change in cellular shape was sodium flux- but only partially NHE1-dependent. In a pathophysiological altered environment, the PAF-induced response of neutrophils was modulated. Acidifying the extracellular pH in vitro enhanced PAF-mediated depolarization, whereas the increases in cell size and intracellular pH were largely unaffected. Ex vivo exposure of human whole blood to lipopolysaccharide diminished the PAF-induced intracellular alkalization and the change in neutrophil size. During experimental porcine sepsis, depolarization of the MP was significantly impaired. Additionally, there was a trend for increased cellular swelling, whereas intracellular alkalization remained stable. Overall, an impaired (electro-)physiological response of neutrophils to PAF stimulation represents a cellular hallmark of those cells challenged during systemic inflammation. Furthermore, this altered response may be indicative of and causative for the development of neutrophil dysfunction during sepsis.
Keywords: flow cytometry; intracellular pH; membrane potential; neutrophil granulocytes; platelet-activating factor; sepsis.
Copyright © 2021 Hug, Bernhard, Stratmann, Erber, Wohlgemuth, Knapp, Bauer, Vidoni, Fauler, Föhr, Radermacher, Hoffmann, Huber-Lang and Messerer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

