IL-1 Family Antagonists in Mouse and Human Skin Inflammation
- PMID: 33796114
- PMCID: PMC8009184
- DOI: 10.3389/fimmu.2021.652846
IL-1 Family Antagonists in Mouse and Human Skin Inflammation
Abstract
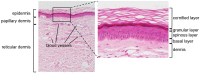
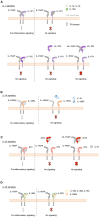
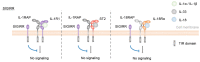
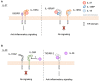
Interleukin (IL)-1 family cytokines initiate inflammatory responses, and shape innate and adaptive immunity. They play important roles in host defense, but excessive immune activation can also lead to the development of chronic inflammatory diseases. Dysregulated IL-1 family signaling is observed in a variety of skin disorders. In particular, IL-1 family cytokines have been linked to the pathogenesis of psoriasis and atopic dermatitis. The biological activity of pro-inflammatory IL-1 family agonists is controlled by the natural receptor antagonists IL-1Ra and IL-36Ra, as well as by the regulatory cytokines IL-37 and IL-38. These four anti-inflammatory IL-1 family members are constitutively and highly expressed at steady state in the epidermis, where keratinocytes are a major producing cell type. In this review, we provide an overview of the current knowledge concerning their regulatory roles in skin biology and inflammation and their therapeutic potential in human inflammatory skin diseases. We further highlight some common misunderstandings and less well-known observations, which persist in the field despite recent extensive interest for these cytokines.
Keywords: atopic dermatitis; cytokine; inflammation; interleukin-1; psoriasis; skin.
Copyright © 2021 Martin, Goldstein, Mermoud, Diaz-Barreiro and Palmer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

