Non-neutralizing Antibodies May Contribute to Suppression of SIVmac239 Viremia in Indian Rhesus Macaques
- PMID: 33796119
- PMCID: PMC8008062
- DOI: 10.3389/fimmu.2021.657424
Non-neutralizing Antibodies May Contribute to Suppression of SIVmac239 Viremia in Indian Rhesus Macaques
Abstract
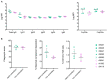
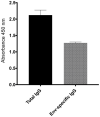
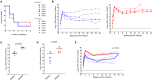
The antiviral properties of broadly neutralizing antibodies against HIV are well-documented but no vaccine is currently able to elicit protective titers of these responses in primates. While current vaccine modalities can readily induce non-neutralizing antibodies against simian immunodeficiency virus (SIV) and HIV, the ability of these responses to restrict lentivirus transmission and replication remains controversial. Here, we investigated the antiviral properties of non-neutralizing antibodies in a group of Indian rhesus macaques (RMs) that were vaccinated with vif, rev, tat, nef, and env, as part of a previous study conducted by our group. These animals manifested rapid and durable control of viral replication to below detection limits shortly after SIVmac239 infection. Although these animals had no serological neutralizing activity against SIVmac239 prior to infection, their pre-challenge titers of Env-binding antibodies correlated with control of viral replication. To assess the contribution of anti-Env humoral immune responses to virologic control in two of these animals, we transiently depleted their circulating antibodies via extracorporeal plasma immunoadsorption and inhibition of IgG recycling through antibody-mediated blockade of the neonatal Fc receptor. These procedures reduced Ig serum concentrations by up to 80% and temporarily induced SIVmac239 replication in these animals. Next, we transferred purified total Ig from the rapid controllers into six vaccinated RMs one day before intrarectal challenge with SIVmac239. Although recipients of the hyperimmune anti-SIV Ig fraction were not protected from infection, their peak and chronic phase viral loads were significantly lower than those in concurrent unvaccinated control animals. Together, our results suggest that non-neutralizing Abs may play a role in the suppression of SIVmac239 viremia.
Keywords: SIV; adoptive transfer; anti-FcRn Ab; antibody depletion; immunoadsorption; non-neutralizing antibodies; rhesus macaques (Macaca mulatta).
Copyright © 2021 Pedreño-Lopez, Rosen, Flores, Gorman, Voigt, Ricciardi, Crosno, Weisgrau, Parks, Lifson, Alter, Rakasz, Magnani, Martins and Watkins.
Conflict of interest statement
JL was employed by Leidos Biomedical Research Inc. MM has a consulting financial interest in Emmune, Inc., a company that is developing HIV immunotherapies based on the immunoadhesin eCD4-Ig. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, Robb ML, et al. . Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys. Lancet. (2018) 392:232–43. 10.1016/S0140-6736(18)31364-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

