The Arabidopsis thaliana E3 Ubiquitin Ligase BRIZ Functions in Abscisic Acid Response
- PMID: 33796126
- PMCID: PMC8008127
- DOI: 10.3389/fpls.2021.641849
The Arabidopsis thaliana E3 Ubiquitin Ligase BRIZ Functions in Abscisic Acid Response
Abstract
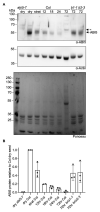
The ubiquitin system is essential for multiple hormone signaling pathways in plants. Here, we show that the Arabidopsis thaliana E3 ligase BRIZ, a heteromeric ligase that consists minimally of BRIZ1 and BRIZ2 proteins, functions in abscisic acid (ABA) signaling or response. briz1 and briz2 homozygous mutants either fail to germinate or emerge later than wild-type seedlings, with little cotyledon expansion or root elongation and no visible greening. Viability staining indicates that briz1 and briz2 embryos are alive but growth-arrested. Germination of briz mutants is improved by addition of the carotenoid biosynthetic inhibitor fluridone or gibberellic acid (GA3), and briz mutants have improved development in backgrounds deficient in ABA synthesis (gin1-3/aba2) or signaling (abi5-7). Endogenous ABA is not higher in briz2 seeds compared to wild-type seeds, and exogenous ABA does not affect BRIZ mRNAs in imbibed seeds. These results indicate that briz embryos are hypersensitive to ABA and that under normal growth conditions, BRIZ acts to suppress ABA signaling or response. ABA signaling and sugar signaling are linked, and we found that briz1 and briz2 mutants excised from seed coats are hypersensitive to sucrose. Although briz single mutants do not grow to maturity, we were able to generate mature briz2-3 abi5-7 double mutant plants that produced seeds. These seeds are more sensitive to exogenous sugar and are larger than seeds from sibling abi5-7 BRIZ2/briz2-3 plants, suggesting that BRIZ has a parental effect on seed development. From these data, we propose a model in which the BRIZ E3 ligase suppresses ABA responses during seed maturation and germination and early seedling establishment.
Keywords: ABA2; ABI5; Arabidopsis; E3 ligase; abscisic acid; germination; hormone signaling; ubiquitin.
Copyright © 2021 Linden, Hsia, Chen and Callis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Barrero J. M., Millar A. A., Griffiths J., Czechowski T., Scheible W. R., Udvardi M., et al. . (2010). Gene expression profiling identifies two regulatory genes controlling dormancy and ABA sensitivity in Arabidopsis seeds. Plant J. 61, 611–622. 10.1111/j.1365-313X.2009.04088.x, PMID: - DOI - PubMed
-
- Barros-Galvão T., Vaistij F. E., Graham I. A. (2019). Control of seed coat rupture by ABA-INSENSITIVE 5 in Arabidopsis thaliana. Seed Sci. Res. 29, 143–148. 10.1017/S0960258519000059 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

