Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases
- PMID: 33796134
- PMCID: PMC8007926
- DOI: 10.3389/fgene.2021.654865
Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases
Abstract
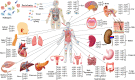
Aquaporins (AQPs) are integral membrane proteins and found in all living organisms from bacteria to human. AQPs mainly involved in the transmembrane diffusion of water as well as various small solutes in a bidirectional manner are widely distributed in various human tissues. Human contains 13 AQPs (AQP0-AQP12) which are divided into three sub-classes namely orthodox aquaporin (AQP0, 1, 2, 4, 5, 6, and 8), aquaglyceroporin (AQP3, 7, 9, and 10) and super or unorthodox aquaporin (AQP11 and 12) based on their pore selectivity. Human AQPs are functionally diverse, which are involved in wide variety of non-infectious diseases including cancer, renal dysfunction, neurological disorder, epilepsy, skin disease, metabolic syndrome, and even cardiac diseases. However, the association of AQPs with infectious diseases has not been fully evaluated. Several studies have unveiled that AQPs can be regulated by microbial and parasitic infections that suggest their involvement in microbial pathogenesis, inflammation-associated responses and AQP-mediated cell water homeostasis. This review mainly aims to shed light on the involvement of AQPs in infectious and non-infectious diseases and potential AQPs-target modulators. Furthermore, AQP structures, tissue-specific distributions and their physiological relevance, functional diversity and regulations have been discussed. Altogether, this review would be useful for further investigation of AQPs as a potential therapeutic target for treatment of infectious as well as non-infectious diseases.
Keywords: aquaporins and infectious diseases; drug targets; functional regulation; human aquaporins; water homeostasis.
Copyright © 2021 Azad, Raihan, Ahmed, Hakim, Emon and Chowdhury.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

