Patterns of treatment and outcome of palbociclib plus endocrine therapy in hormone receptor-positive/HER2 receptor-negative metastatic breast cancer: a real-world multicentre Italian study
- PMID: 33796150
- PMCID: PMC7970542
- DOI: 10.1177/1758835920987651
Patterns of treatment and outcome of palbociclib plus endocrine therapy in hormone receptor-positive/HER2 receptor-negative metastatic breast cancer: a real-world multicentre Italian study
Abstract
Background: The CDK4/6 inhibitor palbociclib combined with endocrine therapy (ET) has proven to prolong progression-free survival (PFS) in women with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (MBC). Few data are available regarding the efficacy of such a regimen outside the clinical trials.
Patients and methods: This is a multicentre prospective real-world experience aimed at verifying the outcome of palbociclib plus ET in an unselected population of MBC patients. The primary aim was the clinical benefit rate (CBR); secondary aims were the median PFS, overall survival (OS) and safety. Patients received palbociclib plus letrozole 2.5 mg (cohort A) or fulvestrant 500 mg (cohort B).
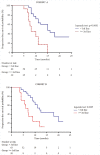
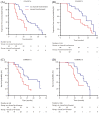
Results: In total, 191 patients (92 in cohort A, 99 in cohort B) were enrolled and treated, and 182 were evaluable for the analysis. Median age was 62 years (range 47-79); 54% had visceral involvement; 28% of patients had previously performed one treatment line (including chemotherapy and ET), 22.6% two lines and 15.9% three. An overall response rate of 34.6% was observed with 11 (6.0%) complete responses and 52 (28.6%) partial responses. Stable disease was achieved by 78 patients (42.9%) with an overall CBR of 59.8%. At a median follow-up of 24 months (range 6-32), median PFS was 13 months without significant differences between the cohorts. When analysed according to treatment line, PFS values were significantly prolonged when palbociclib-based therapy was administered as first-line treatment (14.0 months), to decrease progressively in second and subsequent lines (11.7 and 6.7 months, respectively). Median OS was 25 months, ranging from 28.0 months in 1st line to 18.0 and 13.0 months in 2nd and subsequent lines, respectively.
Conclusions: Our data indicate that palbociclib plus ET is active and safe in HR+/HER2- MBC, also suggesting a better performance of the combinations in earlier treatment lines.
Keywords: endocrine therapy; metastatic breast cancer; palbociclib; real world; visceral involvement.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer 2018; 68: 394–424. - PubMed
-
- National Comprehensive Cancer Network. NCC clinical practice guidelines in oncology. Breast Cancer, Version 4.2020, https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2020, accessed 10 May 2020).
-
- Rugo H, Rumble B, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Cancer Society of Clinical Oncology guideline. J Clin Oncol 2016; 34: 3069–3103. - PubMed
-
- Tryfonidis K, Zardavas D, Katzenellenbogen BS, et al. Endocrine treatment in breast cancer: cure, resistance and beyond. Cancer Treat Rev 2016; 50: 68–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

