Juvenile myelomonocytic leukemia-A comprehensive review and recent advances in management
- PMID: 33796386
- PMCID: PMC8010610
Juvenile myelomonocytic leukemia-A comprehensive review and recent advances in management
Abstract
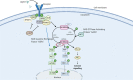
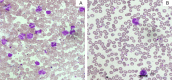
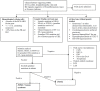
Juvenile myelomonocytic leukemia (JMML) is a rare pediatric myelodysplastic/myeloproliferative neoplasm overlap disease. JMML is associated with mutations in the RAS pathway genes resulting in the myeloid progenitors being sensitive to granulocyte monocyte colony-stimulating factor (GM-CSF). Karyotype abnormalities and additional epigenetic alterations can also be found in JMML. Neurofibromatosis and Noonan's syndrome have a predisposition for JMML. In a few patients, the RAS genes (NRAS, KRAS, and PTPN11) are mutated at the germline and this usually results in a transient myeloproliferative disorder with a good prognosis. JMML with somatic RAS mutation behaves aggressively. JMML presents with cytopenias and leukemic infiltration into organs. The laboratory findings include hyperleukocytosis, monocytosis, increased hemoglobin-F levels, and circulating myeloid precursors. The blast cells in the peripheral blood/bone-marrow aspirate are less than 20% and the absence of the BCR-ABL translocation helps to differentiate from chronic myeloid leukemia. JMML should be differentiated from immunodeficiencies, viral infections, intrauterine infections, hemophagolymphohistiocytosis, other myeloproliferative disorders, and leukemias. Chemotherapy is employed as a bridge to HSCT, except in few with less aggressive disease, in which chemotherapy alone can result in long term remission. Azacitidine has shown promise as a single agent to stabilize the disease. The prognosis of JMML is poor with about 50% of patients surviving after an allogeneic hematopoietic stem cell transplant (HSCT). Allogeneic HSCT is the only known cure for JMML to date. Myeloablative conditioning is most commonly used with graft versus host disease (GVHD) prophylaxis tailored to the aggressiveness of the disease. Relapses are common even after HSCT and a second HSCT can salvage a third of these patients. Novel options in the treatment of JMML e.g., hypomethylating agents, MEK inhibitors, JAK inhibitors, tyrosine kinase inhibitors, etc. are being explored.
Keywords: Juvenile myelomonocytic leukemia; azacitidine; hematopoietic stem cell transplant; monocytosis; mutations; myelodysplastic; myeloproliferative.
AJBR Copyright © 2021.
Conflict of interest statement
AKG has been sanctioned a grant for research on the genomic landscape of JMML from the Indian Council of Medical Research.
Figures
References
-
- Emanuel PD. Juvenile myelomonocytic leukemia. Curr Hematol Rep. 2004;3:203–209. - PubMed
-
- Childhood Acute Myeloid Leukemia/Other Myeloid Malignancies Treatment (PDQ®) - PDQ Cancer Information Summaries - NCBI Bookshelf [Internet]. [cited 2020 Sep 11] Available from: https://www.ncbi.nlm.nih.gov/books/NBK66019/
-
- Cseh A, Niemeyer CM, Yoshimi A, Dworzak M, Hasle H, van den Heuvel-Eibrink MM, Locatelli F, Masetti R, Schmugge M, Groß-Wieltsch U, Candás A, Kulozik AE, Olcay L, Suttorp M, Furlan I, Strahm B, Flotho C. Bridging to transplant with azacitidine in juvenile myelomonocytic leukemia: a retrospective analysis of the EWOG-MDS study group. Blood. 2015;125:2311–2313. - PubMed
-
- Niemeyer CM, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, Haas O, Harbott J, Hasle H, Kerndrup G, Locatelli F, Mann G, Stollmann-Gibbels B, van’t Veer-Korthof ET, van Wering E, Zimmermann M. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) Blood. 1997;89:3534–3543. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
