RIPK3-Dependent Necroptosis Is Induced and Restricts Viral Replication in Human Astrocytes Infected With Zika Virus
- PMID: 33796483
- PMCID: PMC8007970
- DOI: 10.3389/fcimb.2021.637710
RIPK3-Dependent Necroptosis Is Induced and Restricts Viral Replication in Human Astrocytes Infected With Zika Virus
Abstract
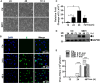
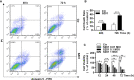
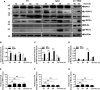
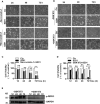
Apoptosis, pyroptosis and necroptosis are regulated processes of cell death which can be crucial for viral disease outcomes in hosts because of their effects on viral pathogenicity and host resistance. Zika virus (ZIKV) is a mosquito-borne flavivirus, which infects humans and can cause neurological disorders. Neural developmental disorders and microcephaly could occur in infected fetuses. Several types of nervous cells have been reported to be susceptible to ZIKV infection. Human astrocytes play important roles in the nutritional support and defense of neurons. In this study, we show that human astrocytes are susceptible to ZIKV infection and undergo progressive cell death after infection. In infected astrocytes we detected no cleavage or activation of pro-caspase-3 and pro-caspase-1. Apoptotic substrates and increased secretion of interleukin (IL)-1β or IL-18 were not detected, either. These ruled out the occurrence of apoptosis or pyroptosis in ZIKV-infected astrocytes. We detected, however, an increase of phosphorylated receptor-interacting serine/threonine-protein kinase (RIPK)1, RIPK3, and mixed lineage kinase domain-like (MLKL) protein, indicating that programmed necrosis, or necroptosis, was induced in infected astrocytes. The phosphorylation and cell death were inhibited in cells pre-treated with GSK'872, an inhibitor of RIPK3, while inhibition of RIPK1 with an inhibitor, Necrostatin-1, had no effect, suggesting that ZIKV-induced necroptosis was RIPK1-independent in astrocytes. Consistent with this finding, the inhibition of RIPK1 had no effect on the phosphorylation of MLKL. We showed evidence that MLKL phosphorylation was RIPK3-dependent and ZBP-1, which could stimulate RIPK3, was upregulated in ZIKV-infected astrocytes. Finally, we demonstrated that in GSK'872-pre-treated astrocytes, viral replication increased significantly, which indicates that necroptosis may be protective against viral replication in astrocytes. Our finding that astrocytes uniquely underwent necroptosis in response to ZIKV infection provides insight and helps us better understand the viral pathogenesis in the ZIKV-infected central nervous system.
Keywords: RIPK1; RIPK3; Zika virus; astrocytes; necroptosis.
Copyright © 2021 Wen, Yu, Gao, Qi, Cardona and Xing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

