LHPP-Mediated Histidine Dephosphorylation Suppresses the Self-Renewal of Mouse Embryonic Stem Cells
- PMID: 33796530
- PMCID: PMC8007871
- DOI: 10.3389/fcell.2021.638815
LHPP-Mediated Histidine Dephosphorylation Suppresses the Self-Renewal of Mouse Embryonic Stem Cells
Abstract
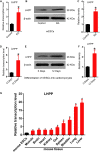
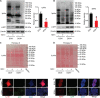
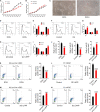
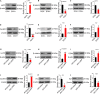
Self-renewal of embryonic stem cells (ESCs) is orchestrated by a vast number of genes at the transcriptional and translational levels. However, the molecular mechanisms of post-translational regulatory factors in ESC self-renewal remain unclear. Histidine phosphorylation, also known as hidden phosphorylation, cannot be detected by conventional experimental methods. A recent study defined phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) as a histidine phosphatase, which regulates various biological behaviors in cells via histidine dephosphorylation. In this study, the doxycycline (DOX)-induced hLHPP-overexpressing mouse ESCs and mouse LHPP silenced mESCs were constructed. Quantitative polymerase chain reaction (qPCR), western blotting analysis, immunofluorescence, Flow cytometry, colony formation assays, alkaline phosphatase (AP) and bromodeoxyuridine (Brdu) staining were performed. We found that the histidine phosphorylation level was strikingly reduced following LHPP overexpression. Besides, the expression of Oct4 and Lefty1, indispensable genes in the process of ESCs self-renewal, was significantly down-regulated, while markers related to the differentiation were markedly elevated. Moreover, LHPP-mediated histidine dephosphorylation induced G0/G1 phase arrest in mESCs, suggesting LHPP was implicated in cell proliferation and cell cycle. Conversely, silencing of Lhpp promoted the self-renewal of mESCs and reversed the RA induced increased expression of genes associated with differentiation. Mechanistically, our findings suggested that the enzymatic active site of LHPP was the cysteine residue at position 226, not 53. LHPP-mediated histidine dephosphorylation lowered the expression levels of β-catenin and the cell cycle-related genes CDK4 and CyclinD1, while it up-regulated the cell cycle suppressor genes P21 and P27. Taken together, our findings reveal that LHPP-mediated histidine dephosphorylation plays a role in the self-renewal of ESCs. LHPP-mediated histidine dephosphorylation inhibited the self-renewal of ESCs by negatively regulating the Wnt/β-catenin pathway and downstream cell cycle-related genes, providing a new perspective and regulatory target for ESCs self-renewal.
Keywords: LHPP; embryonic stem cell; histidine dephosphorylation; post-translational modification; self-renewal.
Copyright © 2021 Xia, Yao, Cai and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Boyer P. D., Deluca M., Ebner K. E., Hultquist D. E., Peter J. B. (1962). Identification of phosphohistidine in digests from a probable intermediate of oxidative phosphorylation. J. Biol. Chem. 237 PC3306–PC3308. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

