Roles of Parathyroid Hormone-Related Protein (PTHrP) and Its Receptor (PTHR1) in Normal and Tumor Tissues: Focus on Their Roles in Osteosarcoma
- PMID: 33796580
- PMCID: PMC8008073
- DOI: 10.3389/fvets.2021.637614
Roles of Parathyroid Hormone-Related Protein (PTHrP) and Its Receptor (PTHR1) in Normal and Tumor Tissues: Focus on Their Roles in Osteosarcoma
Abstract
Osteosarcoma (OS) is the most common primary bone tumor and originates from bone forming mesenchymal cells and primarily affects children and adolescents. The 5-year survival rate for OS is 60 to 65%, with little improvement in prognosis during the last four decades. Studies have demonstrated the evolving roles of parathyroid hormone-related protein (PTHrP) and its receptor (PTHR1) in bone formation, bone remodeling, regulation of calcium transport from blood to milk, regulation of maternal calcium transport to the fetus and reabsorption of calcium in kidneys. These two molecules also play critical roles in the development, progression and metastasis of several tumors such as breast cancer, lung carcinoma, chondrosarcoma, squamous cell carcinoma, melanoma and OS. The protein expression of both PTHrP and PTHR1 have been demonstrated in OS, and their functions and proposed signaling pathways have been investigated yet their roles in OS have not been fully elucidated. This review aims to discuss the latest research with PTHrP and PTHR1 in OS tumorigenesis and possible mechanistic pathways. This review is dedicated to Professor Michael Day who died in May 2020 and was a very generous collaborator.
Keywords: canine; osteosarcoma; parathyroid hormone; parathyroid hormone related protein; prognostic factor.
Copyright © 2021 Al-Khan, Al Balushi, Richardson and Danks.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
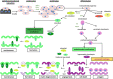
 , pre-osteoblasts
, pre-osteoblasts  , chondroblasts
, chondroblasts  , chondrocytes
, chondrocytes  , osteoclasts
, osteoclasts  , hypertrophic chondrocytes
, hypertrophic chondrocytes  , adipocytes
, adipocytes  and myocytes
and myocytes  . Reproduced with permission from reference (59).
. Reproduced with permission from reference (59).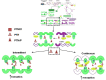
 and PTH
and PTH  are given intermittently to patients, they increase the formation of new bone
are given intermittently to patients, they increase the formation of new bone  but if either are given continuously, they increase resorption by stimulating osteoclasts to remodel bone
but if either are given continuously, they increase resorption by stimulating osteoclasts to remodel bone  . Both act via PTHR1
. Both act via PTHR1  . Reproduced with permission from reference (59).
. Reproduced with permission from reference (59).
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

