Live Intravital Intestine with Blood Flow Visualization in Neonatal Mice Using Two-photon Laser Scanning Microscopy
- PMID: 33796611
- PMCID: PMC8005880
- DOI: 10.21769/BioProtoc.3937
Live Intravital Intestine with Blood Flow Visualization in Neonatal Mice Using Two-photon Laser Scanning Microscopy
Abstract
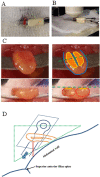
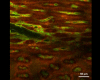
This protocol describes a novel technique to investigate the microcirculation dynamics underlying the pathology in the small intestine of neonatal mice using two-photon laser-scanning microscopy (TPLSM). Recent technological advances in multi-photon microscopy allow intravital analysis of different organs such as the liver, brain and intestine. Despite these advances, live visualization and analysis of the small intestine in neonatal rodents remain technically challenging. We herein provide a detailed description of a novel method to capture high resolution and stable images of the small intestine in neonatal mice as early as postnatal day 0. This imaging technique allows a comprehensive understanding of the development and blood flow dynamics in small intestine microcirculation.
Keywords: In vivo imaging; Intravital imaging; Necrotizing enterocolitis (NEC); Neonatal mouse imaging; Two-photon laser scanning microscopy (TPLSM).
Copyright © 2021 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe authors have no conflict of interest to declare.
Figures





References
-
- Barlow B., Santulli T. V., Heird W. C., Pitt J., Blanc W. A. and Schullinger J. N.(1974). An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg 9(5): 587-595. - PubMed
-
- Devi S., Li A., Westhorpe C. L., Lo C. Y., Abeynaike L. D., Snelgrove S. L., Hall P., Ooi J. D., Sobey C. G., Kitching A. R. and Hickey M. J.(2013). Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med 19(1): 107-112. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

