Three-dimensional understanding of the morphological complexity of the human uterine endometrium
- PMID: 33796844
- PMCID: PMC7995615
- DOI: 10.1016/j.isci.2021.102258
Three-dimensional understanding of the morphological complexity of the human uterine endometrium
Abstract
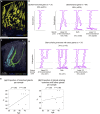
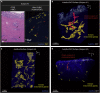
The fundamental morphology of the endometrial glands is not sufficiently understood by 2D observation because these glands have complicated winding and branching patterns. To construct a large picture of the endometrial gland structure, we performed tissue-clearing-based 3D imaging of human uterine endometrial tissue. Our 3D immunohistochemistry and layer analyses revealed that the endometrial glands form a plexus network in the stratum basalis and expand horizontally along the muscular layer, similar to the rhizome of grass. We then extended our method to assess the 3D morphology of tissue affected by adenomyosis, a representative "endometrium-related disease," and observed its 3D morphological features, including the direct invasion of endometrial glands into the myometrium and an ant colony-like network of ectopic endometrial glands within the myometrium. Thus, further understanding of the morphology of the human endometrium based on 3D analysis will lead to the identification of the pathogenesis of endometrium-related diseases.
Keywords: Human Physiology; Imaging Anatomy.
© 2021 The Author(s).
Conflict of interest statement
RIKEN Quantitative Biology Center has filed a patent based on this work in which K. Tainaka is a co-inventor. Other authors declare no competing interests.
Figures






References
-
- Al-Hussaini M., Ashi S.A.-L., Ardighieri L., Ayhan A., Bennett J., Desouk M.M., Garcia R., Gilks B., Han L., Haque M. Uterus. In Pathology Outlines.com. 2020. https://www.pathologyoutlines.com/uterus.html
-
- Benagiano G., Brosens I. History of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006;20:449–463. - PubMed
-
- Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004;70:1738–1750. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

