Fronto-temporal dementia risk gene TMEM106B has opposing effects in different lysosomal storage disorders
- PMID: 33796852
- PMCID: PMC7990118
- DOI: 10.1093/braincomms/fcaa200
Fronto-temporal dementia risk gene TMEM106B has opposing effects in different lysosomal storage disorders
Abstract
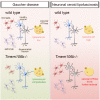
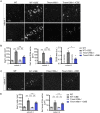
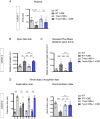
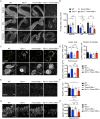
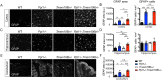
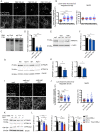
TMEM106B is a transmembrane protein localized to the endo-lysosomal compartment. Genome-wide association studies have identified TMEM106B as a risk modifier of Alzheimer's disease and frontotemporal lobar degeneration, especially with progranulin haploinsufficiency. We recently demonstrated that TMEM106B loss rescues progranulin null mouse phenotypes including lysosomal enzyme dysregulation, neurodegeneration and behavioural alterations. However, the reason whether TMEM106B is involved in other neurodegenerative lysosomal diseases is unknown. Here, we evaluate the potential role of TMEM106B in modifying the progression of lysosomal storage disorders using progranulin-independent models of Gaucher disease and neuronal ceroid lipofuscinosis. To study Gaucher disease, we employ a pharmacological approach using the inhibitor conduritol B epoxide in wild-type and hypomorphic Tmem106b-/- mice. TMEM106B depletion ameliorates neuronal degeneration and some behavioural abnormalities in the pharmacological model of Gaucher disease, similar to its effect on certain progranulin null phenotypes. In order to examine the role of TMEM106B in neuronal ceroid lipofuscinosis, we crossbred Tmem106b-/- mice with Ppt1-/-, a genetic model of the disease. In contrast to its conduritol B epoxide-rescuing effect, TMEM106B loss exacerbates Purkinje cell degeneration and motor deficits in Ppt1-/- mice. Mechanistically, TMEM106B is known to interact with subunits of the vacuolar ATPase and influence lysosomal acidification. In the pharmacological Gaucher disease model, the acidified lysosomal compartment is enhanced and TMEM106B loss rescues in vivo phenotypes. In contrast, gene-edited neuronal loss of Ppt1 causes a reduction in vacuolar ATPase levels and impairment of the acidified lysosomal compartment, and TMEM106B deletion exacerbates the mouse Ppt1-/- phenotype. Our findings indicate that TMEM106B differentially modulates the progression of the lysosomal storage disorders Gaucher disease and neuronal ceroid lipofuscinosis. The effect of TMEM106B in neurodegeneration varies depending on vacuolar ATPase state and modulation of lysosomal pH. These data suggest TMEM106B as a target for correcting lysosomal pH alterations, and in particular for therapeutic intervention in Gaucher disease and neuronal ceroid lipofuscinosis.
Keywords: Gaucher; TMEM106B; lysosome; neuronal ceroid lipofuscinosis; palmitoyl-protein thioesterase 1.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Česen M, Pegan K, Špes A, Turk B.. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res 2012; 318: 1245–51. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
