ISG15 is downregulated by KLF12 and implicated in maintenance of cancer stem cell-like features in cisplatin-resistant ovarian cancer
- PMID: 33797839
- PMCID: PMC8093991
- DOI: 10.1111/jcmm.16503
ISG15 is downregulated by KLF12 and implicated in maintenance of cancer stem cell-like features in cisplatin-resistant ovarian cancer
Abstract
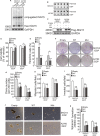
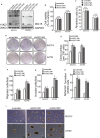
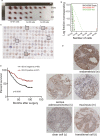
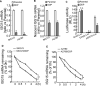
Drug resistance is often developed during clinical chemotherapy of ovarian cancers. The ubiquitin-like protein interferon-stimulated gene 15 (ISG15) is possibly dependent on tumour context to promote or suppress progression of various tumours. The ubiquitin-like protein interferon-stimulated gene 15 (ISG15) was decreased in cisplatin-resistant ovarian cancer cells. The current study identified that both ectopic wild type and nonISGylatable mutant ISG15 expression inhibited CSC-like phenotypes of cisplatin-resistant ovarian cancer cells. Moreover, ectopic ISG15 expression suppressed tumour formation in nude mice. In addition, ISG15 downregulation promoted CSC-like features of cisplatin-sensitive ovarian cancer cells. Furthermore, low ISG15 expression was associated with poor prognosis in patients with ovarian cancer. Transcriptional repressor Krüppel-like factor 12 (KLF12) downregulated ISG15 in cisplatin-resistant cells. Our data indicated that downregulating ISG15 expression, via weakening effect of KLF12, might be considered as new therapeutic strategy to inhibit CSC phenotypes in the treatment of cisplatin-resistant ovarian cancer.
Keywords: ISG15; KLF12; cancer stem cell; cisplatin resistance; ovarian cancer.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
ISG15 suppresses translation of ABCC2 via ISGylation of hnRNPA2B1 and enhances drug sensitivity in cisplatin resistant ovarian cancer cells.Biochim Biophys Acta Mol Cell Res. 2020 Apr;1867(4):118647. doi: 10.1016/j.bbamcr.2020.118647. Epub 2020 Jan 9. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31926942
-
Downregulation of MYPT1 increases tumor resistance in ovarian cancer by targeting the Hippo pathway and increasing the stemness.Mol Cancer. 2020 Jan 11;19(1):7. doi: 10.1186/s12943-020-1130-z. Mol Cancer. 2020. PMID: 31926547 Free PMC article.
-
FOXP1 functions as an oncogene in promoting cancer stem cell-like characteristics in ovarian cancer cells.Oncotarget. 2016 Jan 19;7(3):3506-19. doi: 10.18632/oncotarget.6510. Oncotarget. 2016. PMID: 26654944 Free PMC article.
-
Regulation and action of interferon-stimulated gene 15 in breast cancer cells.Hum Cell. 2020 Oct;33(4):954-962. doi: 10.1007/s13577-020-00414-x. Epub 2020 Aug 19. Hum Cell. 2020. PMID: 32813218 Review.
-
Regulation of ovarian cancer stem cells or tumor-initiating cells.Int J Mol Sci. 2013 Mar 25;14(4):6624-48. doi: 10.3390/ijms14046624. Int J Mol Sci. 2013. PMID: 23528891 Free PMC article. Review.
Cited by
-
Interferon‑stimulated gene 15 promotes progression of endometrial carcinoma and weakens antitumor immune response.Oncol Rep. 2022 Jun;47(6):110. doi: 10.3892/or.2022.8321. Epub 2022 Apr 21. Oncol Rep. 2022. PMID: 35445736 Free PMC article.
-
Interferon-stimulated gene subtypes as key indicators of immune landscape and survival outcomes in ovarian cancer.Discov Oncol. 2024 Dec 18;15(1):775. doi: 10.1007/s12672-024-01617-6. Discov Oncol. 2024. PMID: 39692913 Free PMC article.
-
Krüppel-like factor 12 decreases progestin sensitivity in endometrial cancer by inhibiting the progesterone receptor signaling pathway.Transl Oncol. 2024 Sep;47:102041. doi: 10.1016/j.tranon.2024.102041. Epub 2024 Jul 2. Transl Oncol. 2024. PMID: 38959708 Free PMC article.
-
The role of interferons in ovarian cancer progression: Hinderer or promoter?Front Immunol. 2022 Dec 21;13:1087620. doi: 10.3389/fimmu.2022.1087620. eCollection 2022. Front Immunol. 2022. PMID: 36618371 Free PMC article. Review.
-
Transcriptome-wide N6-methyladenine methylation in granulosa cells of women with decreased ovarian reserve.BMC Genomics. 2022 Mar 28;23(1):240. doi: 10.1186/s12864-022-08462-3. BMC Genomics. 2022. PMID: 35346019 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

