Phosphonofluoresceins: Synthesis, Spectroscopy, and Applications
- PMID: 33797899
- PMCID: PMC8327618
- DOI: 10.1021/jacs.1c01139
Phosphonofluoresceins: Synthesis, Spectroscopy, and Applications
Abstract
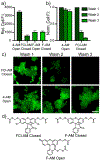
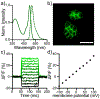
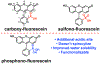
Xanthene fluorophores, like fluorescein, have been versatile molecules across diverse fields of chemistry and life sciences. Despite the ubiquity of 3-carboxy and 3-sulfonofluorescein for the last 150 years, to date, no reports of 3-phosphonofluorescein exist. Here, we report the synthesis, spectroscopic characterization, and applications of 3-phosphonofluoresceins. The absorption and emission of 3-phosphonofluoresceins remain relatively unaltered from the parent 3-carboxyfluorescein. 3-Phosphonofluoresceins show enhanced water solubility compared to 3-carboxyfluorescein and persist in an open, visible light-absorbing state even at low pH and in low dielectric media while 3-carboxyfluoresceins tend to lactonize. In contrast, the spirocyclization tendency of 3-phosphonofluoresceins can be modulated by esterification of the phosphonic acid. The bis-acetoxymethyl ester of 3-phosphonofluorescein readily enters living cells, showing excellent accumulation (>6x) and retention (>11x), resulting in a nearly 70-fold improvement in cellular brightness compared to 3-carboxyfluorescein. In a complementary fashion, the free acid form of 3-phosphonofluorescein does not cross cellular membranes, making it ideally suited for incorporation into a voltage-sensing scaffold. We develop a new synthetic route to functionalized 3-phosphonofluoresceins to enable the synthesis of phosphono-voltage sensitive fluorophores, or phosVF2.1.Cl. Phosphono-VF2.1.Cl shows excellent membrane localization, cellular brightness, and voltage sensitivity (26% ΔF/F per 100 mV), rivaling that of sulfono-based VF dyes. In summary, we develop the first synthesis of 3-phosphonofluoresceins, characterize the spectroscopic properties of this new class of xanthene dyes, and utilize these insights to show the utility of 3-phosphonofluoresceins in intracellular imaging and membrane potential sensing.
Figures


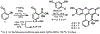


References
-
- Baeyer A, Ueber eine neue Klasse von Farbstoffen. Berichte der deutschen chemischen Gesellschaft 1871, 4 (2), 555–558.
-
- Coons AH; Creech HJ; Jones RN; Berliner E, The Demonstration of Pneumococcal Antigen in Tissues by the Use of Fluorescent Antibody. The Journal of Immunology 1942, 45 (3), 159.
-
- Clark Brelje T; Wessendorf MW; Sorenson RL, Chapter 5 - Multicolor Laser Scanning Confocal Immunofluorescence Microscopy: Practical Application and Limitations. In Methods in Cell Biology, Matsumoto B, Academic Press: 2002; Vol. 70, pp 165–249e. - PubMed
-
- Wilson SA; Last A, Management of corneal abrasions. American family physician 2004, 70 (1), 123–8. - PubMed
-
- Marmor MF; Ravin JG, Fluorescein angiography: insight and serendipity a half century ago. Archives of ophthalmology (Chicago, Ill. : 1960) 2011, 129 (7), 943–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

