Effect of Delayed-Release and Extended-Release Methylphenidate on Caregiver Strain and Validation of Psychometric Properties of the Caregiver Strain Questionnaire: Results from a Phase 3 Trial in Children with Attention-Deficit/Hyperactivity Disorder
- PMID: 33797983
- PMCID: PMC8066344
- DOI: 10.1089/cap.2020.0159
Effect of Delayed-Release and Extended-Release Methylphenidate on Caregiver Strain and Validation of Psychometric Properties of the Caregiver Strain Questionnaire: Results from a Phase 3 Trial in Children with Attention-Deficit/Hyperactivity Disorder
Abstract
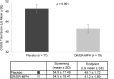
Objectives: Inadequately controlled symptoms and associated impaired functioning have a significant negative impact on caregivers of children with attention-deficit/hyperactivity disorder (ADHD). This study aimed to assess the impact of evening-dosed, delayed-release and extended-release methylphenidate (DR/ER-MPH) treatment on caregiver strain, measured by the Caregiver Strain Questionnaire (CGSQ), and present post hoc psychometric analyses assessing the reliability and validity of the CGSQ, its ability to detect change (responsiveness), and to derive responder definitions. Methods: The CGSQ was an exploratory efficacy endpoint in a phase 3, 3-week, randomized, double-blind, multicenter, placebo-controlled, forced-dose titration trial of DR/ER-MPH in children aged 6-12 years with ADHD (NCT02520388). Psychometric properties of the CGSQ evaluated post hoc included internal consistency using Cronbach's alpha; test/retest reliability using intraclass correlation coefficients (ICCs); construct validity (known groups and convergent/divergent validity); responsiveness to changes in assessments of ADHD severity (ADHD Rating Scale-IV [ADHD-RS-IV], Conners' Global Index-Parent [CGI-P], and Clinical Global Impression-Severity [CGI-S]/CGI-Improvement [CGI-I]); and meaningful change threshold (MCT) using receiver operating characteristic curves, which were used to compare response between DR/ER-MPH and placebo groups. Results: Randomized DR/ER-MPH (54.5) and placebo (54.9) groups had similar mean CGSQ scores at screening. Caregivers of children on DR/ER-MPH reported significant reductions in CGSQ scores after 3 weeks of DR/ER-MPH treatment versus placebo (least-squares mean: 41.2 vs. 49.1; p < 0.001). The CGSQ demonstrated strong internal consistency (Cronbach's alpha = 0.93) and good test/retest reliability (ICC = 0.72). Known groups, convergent/divergent validity, and responsiveness were demonstrated from relationships between the CGSQ and the CGI-S, ADHD-RS-IV, and CGI-P. The mean anchor-based MCT for CGSQ total score was estimated as -9.0 (DR/ER-MPH vs. placebo: 53.2% vs. 29.9% p = 0.003). Conclusions: CGSQ scores significantly decreased after 3 weeks of DR/ER-MPH treatment versus placebo, and the CGSQ was found to be a valid and reliable measure of strain in caregivers of children with ADHD. Clinical trial registration identification number: NCT02520388.
Keywords: DR/ER-MPH; HLD200; caregiver strain; methylphenidate; psychometrics; validation.
Conflict of interest statement
F.A.L. has served as a consultant to and received speaker fees and/or research support from Eli Lilly, GSK, Ironshore, Neos, Novartis, Noven, Pfizer, Shire, Sunovion, Supernus, and Tris. S.V.F. received income, potential income, travel expenses, continuing education support, and/or research support from Takeda, OnDosis, Tris, Otsuka, Arbor, Ironshore, Rhodes, Akili Interactive Labs, Sunovion, Supernus, and Genomind. With his institution, he has US patent US20130217707 A1 for the use of sodium/hydrogen exchange inhibitors in the treatment of ADHD. He also receives royalties from books published by Guilford Press:
Figures
References
-
- Aldridge VK, Dovey TM, Wade A: Assessing test-retest reliability of psychological measures: Persistent methodological problems. Eur Psychol 22:207–218, 2017
-
- American Psychiatric Association: DSM 5 Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (APA); 2013
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical