Cryopreserved Spontaneous Spheroids from Compact Bone-Derived Mesenchymal Stromal Cells for Bone Tissue Engineering
- PMID: 33798009
- PMCID: PMC8064946
- DOI: 10.1089/ten.TEC.2021.0001
Cryopreserved Spontaneous Spheroids from Compact Bone-Derived Mesenchymal Stromal Cells for Bone Tissue Engineering
Abstract
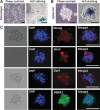
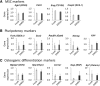
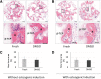
Spontaneously formed spheroids from mouse compact bone-derived mesenchymal stromal cells (CB-MSCs) possess enhanced stemness and superior plasticity. In this study, the effect of cryopreservation on viability, stemness, and osteogenic differentiation capability of spontaneous CB-MSC spheroids were investigated. CB-MSCs were isolated from mouse femur and tibia. Spheroids were cryopreserved with various concentrations of dimethyl sulfoxide (DMSO). After thawing, the number of living and dead cells was measured. The expression levels of stem cell markers and osteogenic marker genes were analyzed. The cryopreserved and noncryopreserved spheroids were transplanted in mice with a beta-tricalcium phosphate as a scaffold to evaluate the in vivo bone-forming capability. The percentage of living cells was highest when 5% DMSO was used as a cryoprotectant, confirmed by the number of dead cells. The expression of stem cell marker genes and osteogenic differentiation capability were maintained after cryopreservation with 5% DMSO. The cryopreserved spontaneous CB-MSC spheroids showed remarkable new bone formation in vivo, identical to that of the noncryopreserved spheroids even without osteogenic induction. The cryopreserved spontaneous CB-MSC spheroids retained stemness and osteogenic differentiation capability and highlight the utility of spontaneous CB-MSC spheroids as ready-to-use tissue-engineered products for bone tissue engineering.
Keywords: MSCs; compact bone-derived mesenchymal stromal cells; cryopreservation; osteogenic differentiation capability; spontaneous spheroid.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Spontaneous spheroids from alveolar bone-derived mesenchymal stromal cells maintain pluripotency of stem cells by regulating hypoxia-inducible factors.Biol Res. 2023 Apr 5;56(1):17. doi: 10.1186/s40659-023-00421-w. Biol Res. 2023. PMID: 37016436 Free PMC article.
-
Spontaneous Spheroids of hUC-MSCs Regulate Osteogenic Differentiation for Enhancing Osteogenesis.Tissue Eng Part C Methods. 2025 Mar;31(3):108-118. doi: 10.1089/ten.tec.2024.0297. Epub 2025 Mar 10. Tissue Eng Part C Methods. 2025. PMID: 40062549
-
Cryopreserved clumps of mesenchymal stem cell/extracellular matrix complexes retain osteogenic capacity and induce bone regeneration.Stem Cell Res Ther. 2018 Mar 21;9(1):73. doi: 10.1186/s13287-018-0826-0. Stem Cell Res Ther. 2018. PMID: 29562931 Free PMC article.
-
Mesenchymal stem cell-associated lncRNA in osteogenic differentiation.Biomed Pharmacother. 2019 Jul;115:108912. doi: 10.1016/j.biopha.2019.108912. Epub 2019 Apr 29. Biomed Pharmacother. 2019. PMID: 31048188 Review.
-
Comparison of freshly cultured versus cryopreserved mesenchymal stem cells in animal models of inflammation: A pre-clinical systematic review.Elife. 2022 Jul 15;11:e75053. doi: 10.7554/eLife.75053. Elife. 2022. PMID: 35838024 Free PMC article.
Cited by
-
Spheroids and organoids: Their implications for oral and craniofacial tissue/organ regeneration.J Oral Biol Craniofac Res. 2024 Sep-Oct;14(5):540-546. doi: 10.1016/j.jobcr.2024.07.002. Epub 2024 Jul 9. J Oral Biol Craniofac Res. 2024. PMID: 39092136 Free PMC article.
-
Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation.Bioengineering (Basel). 2023 Feb 6;10(2):211. doi: 10.3390/bioengineering10020211. Bioengineering (Basel). 2023. PMID: 36829705 Free PMC article. Review.
-
Tissue Engineering with Compact Bone-Derived Cell Spheroids Enables Bone Formation around Transplanted Tooth.Tissue Eng Regen Med. 2022 Apr;19(2):377-387. doi: 10.1007/s13770-021-00423-3. Epub 2022 Feb 4. Tissue Eng Regen Med. 2022. PMID: 35119647 Free PMC article.
References
-
- De Luca, M., Aiuti, A., Cossu, G., et al. . Advances in stem cell research and therapeutic development. Nat Cell Biol 21, 801, 2019 - PubMed
-
- Viswanathan, S., Shi, Y., Galipeau, J., et al. . Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 21, 1019, 2019 - PubMed
-
- Moll, G., Ankrum, J.A., Kamhieh-Milz, J., et al. . Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med 25, 149, 2019 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
