Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial
- PMID: 33798499
- PMCID: PMC8009612
- DOI: 10.1016/S0140-6736(21)00628-0
Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial
Abstract
Background: A new variant of SARS-CoV-2, B.1.1.7, emerged as the dominant cause of COVID-19 disease in the UK from November, 2020. We report a post-hoc analysis of the efficacy of the adenoviral vector vaccine, ChAdOx1 nCoV-19 (AZD1222), against this variant.
Methods: Volunteers (aged ≥18 years) who were enrolled in phase 2/3 vaccine efficacy studies in the UK, and who were randomly assigned (1:1) to receive ChAdOx1 nCoV-19 or a meningococcal conjugate control (MenACWY) vaccine, provided upper airway swabs on a weekly basis and also if they developed symptoms of COVID-19 disease (a cough, a fever of 37·8°C or higher, shortness of breath, anosmia, or ageusia). Swabs were tested by nucleic acid amplification test (NAAT) for SARS-CoV-2 and positive samples were sequenced through the COVID-19 Genomics UK consortium. Neutralising antibody responses were measured using a live-virus microneutralisation assay against the B.1.1.7 lineage and a canonical non-B.1.1.7 lineage (Victoria). The efficacy analysis included symptomatic COVID-19 in seronegative participants with a NAAT positive swab more than 14 days after a second dose of vaccine. Participants were analysed according to vaccine received. Vaccine efficacy was calculated as 1 - relative risk (ChAdOx1 nCoV-19 vs MenACWY groups) derived from a robust Poisson regression model. This study is continuing and is registered with ClinicalTrials.gov, NCT04400838, and ISRCTN, 15281137.
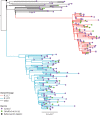
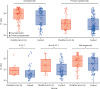
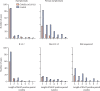
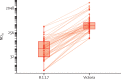
Findings: Participants in efficacy cohorts were recruited between May 31 and Nov 13, 2020, and received booster doses between Aug 3 and Dec 30, 2020. Of 8534 participants in the primary efficacy cohort, 6636 (78%) were aged 18-55 years and 5065 (59%) were female. Between Oct 1, 2020, and Jan 14, 2021, 520 participants developed SARS-CoV-2 infection. 1466 NAAT positive nose and throat swabs were collected from these participants during the trial. Of these, 401 swabs from 311 participants were successfully sequenced. Laboratory virus neutralisation activity by vaccine-induced antibodies was lower against the B.1.1.7 variant than against the Victoria lineage (geometric mean ratio 8·9, 95% CI 7·2-11·0). Clinical vaccine efficacy against symptomatic NAAT positive infection was 70·4% (95% CI 43·6-84·5) for B.1.1.7 and 81·5% (67·9-89·4) for non-B.1.1.7 lineages.
Interpretation: ChAdOx1 nCoV-19 showed reduced neutralisation activity against the B.1.1.7 variant compared with a non-B.1.1.7 variant in vitro, but the vaccine showed efficacy against the B.1.1.7 variant of SARS-CoV-2.
Funding: UK Research and Innovation, National Institute for Health Research (NIHR), Coalition for Epidemic Preparedness Innovations, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midlands NIHR Clinical Research Network, and AstraZeneca.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. AstraZeneca reviewed the data from the study and the final manuscript before submission but the authors retained editorial control. SCG is cofounder of Vaccitech (collaborators in the early development of this vaccine candidate) and is named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and a patent application covering this SARS-CoV-2 vaccine. TL is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and was a consultant to Vaccitech. PMF is a consultant to Vaccitech. AJP is chair of the UK Department of Health and Social Care Joint Committee on Vaccination and Immunisation but does not participate in policy advice on coronavirus vaccines, and is a member of the WHO Strategic Advisory Group of Experts. AJP and SNF are NIHR senior investigators. AVSH is a cofounder of and consultant to Vaccitech and is named as an inventor on a patent covering design and use of ChAdOx1-vectored vaccines (PCT/GB2012/000467). MDS reports grants from Janssen, GlaxoSmithKline, Medimmune, Novavax, and MCM Vaccine, and grants and non-financial support from Pfizer outside of the submitted work. CMG reports personal fees from the Duke Human Vaccine Institute outside of the submitted work. ADD reports grants and personal fees from AstraZeneca outside of the submitted work. SNF reports grants from Janssen and Valneva outside of the submitted work. TLV and JV are employees of AstraZeneca. All other authors declare no competing interests.
Figures


Comment in
-
Pandemic moves and countermoves: vaccines and viral variants.Lancet. 2021 Apr 10;397(10282):1326-1327. doi: 10.1016/S0140-6736(21)00730-3. Epub 2021 Mar 30. Lancet. 2021. PMID: 33798497 Free PMC article. No abstract available.
References
-
- Novavax Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. Jan 28, 2021. https://ir.novavax.com/news-releases/news-release-details/novavax-covid-...
-
- Janssen Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. Jan 29, 2021. https://www.janssen.com/johnson-johnson-announces-single-shot-janssen-co...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

