IL-4Rα signaling by CD8α+ dendritic cells contributes to cerebral malaria by enhancing inflammatory, Th1, and cytotoxic CD8+ T cell responses
- PMID: 33798555
- PMCID: PMC8100064
- DOI: 10.1016/j.jbc.2021.100615
IL-4Rα signaling by CD8α+ dendritic cells contributes to cerebral malaria by enhancing inflammatory, Th1, and cytotoxic CD8+ T cell responses
Abstract
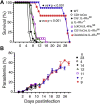
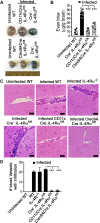
Persistent high levels of proinflammatory and Th1 responses contribute to cerebral malaria (CM). Suppression of inflammatory responses and promotion of Th2 responses prevent pathogenesis. IL-4 commonly promotes Th2 responses and inhibits inflammatory and Th1 responses. Therefore, IL-4 is widely considered as a beneficial cytokine via its Th2-promoting role that is predicted to provide protection against severe malaria by inhibiting inflammatory responses. However, IL-4 may also induce inflammatory responses, as the result of IL-4 action depends on the timing and levels of its production and the tissue environment in which it is produced. Recently, we showed that dendritic cells (DCs) produce IL-4 early during malaria infection in response to a parasite protein and that this IL-4 response may contribute to severe malaria. However, the mechanism by which IL-4 produced by DCs contributing to lethal malaria is unknown. Using Plasmodium berghei ANKA-infected C57BL/6 mice, a CM model, we show here that mice lacking IL-4Rα only in CD8α+ DCs are protected against CM pathogenesis and survive, whereas WT mice develop CM and die. Compared with WT mice, mice lacking IL-4Rα in CD11c+ or CD8α+ DCs showed reduced inflammatory responses leading to decreased Th1 and cytotoxic CD8+ T cell responses, lower infiltration of CD8+ T cells to the brain, and negligible brain pathology. The novel results presented here reveal a paradoxical role of IL-4Rα signaling in CM pathogenesis that promotes CD8α+ DC-mediated inflammatory responses that generate damaging Th1 and cytotoxic CD8+ T cell responses.
Keywords: IL-4Rα; cerebral malaria; cytotoxic T cells; endothelial damage; infiltration to brain; inflammatory cytokines.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- de Souza M.C., Pádua T.A., das Graças Henriques M. Multiple organ dysfunction during severe malaria: The role of the inflammatory response. In: Rodriguez-Marales A.J., editor. Current Topics in Malaria. InTech Publishing; Rijeka, Croatia: 2016.
-
- Hunt N.H., Grau G.E. Cytokines: Accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. - PubMed
-
- Deroost K., Pham T.-T., Opdenakker G., Van den Steen P.E. The immunological balance between host and parasite in malaria. FEMS Microbiol. Rev. 2015;40:208–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

