Compound- and fiber type-selective requirement of AMPKγ3 for insulin-independent glucose uptake in skeletal muscle
- PMID: 33798773
- PMCID: PMC8381060
- DOI: 10.1016/j.molmet.2021.101228
Compound- and fiber type-selective requirement of AMPKγ3 for insulin-independent glucose uptake in skeletal muscle
Abstract
Objective: The metabolic master-switch AMP-activated protein kinase (AMPK) mediates insulin-independent glucose uptake in muscle and regulates the metabolic activity of brown and beige adipose tissue (BAT). The regulatory AMPKγ3 isoform is uniquely expressed in skeletal muscle and potentially in BAT. Herein, we investigated the role that AMPKγ3 plays in mediating skeletal muscle glucose uptake and whole-body glucose clearance in response to small-molecule activators that act on AMPK via distinct mechanisms. We also assessed whether γ3 plays a role in adipose thermogenesis and browning.
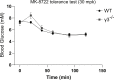
Methods: Global AMPKγ3 knockout (KO) mice were generated. A systematic whole-body, tissue, and molecular phenotyping linked to glucose homeostasis was performed in γ3 KO and wild-type (WT) mice. Glucose uptake in glycolytic and oxidative skeletal muscle ex vivo as well as blood glucose clearance in response to small molecule AMPK activators that target the nucleotide-binding domain of the γ subunit (AICAR) and allosteric drug and metabolite (ADaM) site located at the interface of the α and β subunit (991, MK-8722) were assessed. Oxygen consumption, thermography, and molecular phenotyping with a β3-adrenergic receptor agonist (CL-316,243) treatment were performed to assess BAT thermogenesis, characteristics, and function.
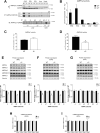
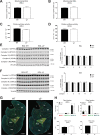
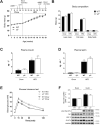
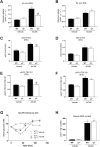
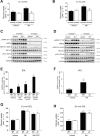
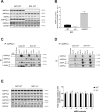
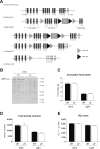
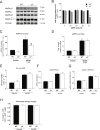
Results: Genetic ablation of γ3 did not affect body weight, body composition, physical activity, and parameters associated with glucose homeostasis under chow or high-fat diet. γ3 deficiency had no effect on fiber-type composition, mitochondrial content and components, or insulin-stimulated glucose uptake in skeletal muscle. Glycolytic muscles in γ3 KO mice showed a partial loss of AMPKα2 activity, which was associated with reduced levels of AMPKα2 and β2 subunit isoforms. Notably, γ3 deficiency resulted in a selective loss of AICAR-, but not MK-8722-induced blood glucose-lowering in vivo and glucose uptake specifically in glycolytic muscle ex vivo. We detected γ3 in BAT and found that it preferentially interacts with α2 and β2. We observed no differences in oxygen consumption, thermogenesis, morphology of BAT and inguinal white adipose tissue (iWAT), or markers of BAT activity between WT and γ3 KO mice.
Conclusions: These results demonstrate that γ3 plays a key role in mediating AICAR- but not ADaM site binding drug-stimulated blood glucose clearance and glucose uptake specifically in glycolytic skeletal muscle. We also showed that γ3 is dispensable for β3-adrenergic receptor agonist-induced thermogenesis and browning of iWAT.
Keywords: 5-aminoimidazole-4-carboxamide riboside; AMP-activated protein kinase; Beige adipose tissue; Brown adipose tissue; MK-8722; TBC1D1.
Copyright © 2021 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Hawley S.A., Davison M., Woods A., Davies S.P., Beri R.K., Carling D. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. Journal of Biological Chemistry. 1996;271(44):27879–27887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

