Nanostructured TiO2 cavitation agents for dual-modal sonophotocatalysis with pulsed ultrasound
- PMID: 33799108
- PMCID: PMC8044705
- DOI: 10.1016/j.ultsonch.2021.105530
Nanostructured TiO2 cavitation agents for dual-modal sonophotocatalysis with pulsed ultrasound
Abstract
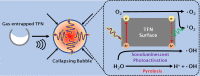
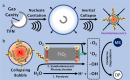
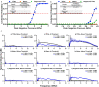
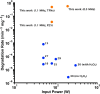
Current sonochemical methods rely on spatially uncontrolled cavitation for radical species generation to promote chemical reactions. To improve radical generation, sonosensitizers have been demonstrated to be activated by cavitation-based light emission (sonoluminescence). Unfortunately, this process remains relatively inefficient compared to direct photocatalysis, due to the physical separation between cavitation event and sonosensitizing agent. In this study, we have synthesized nanostructured titanium dioxide particles to couple the source for cavitation within a photocatalytic site to create a sonophotocatalyst. In doing so, we demonstrate that site-controlled cavitation from the nanoparticles using pulsed ultrasound at reduced acoustic powers resulted in the sonochemical degradation methylene blue at rates nearly three orders of magnitude faster than other titanium dioxide-based nanoparticles by conventional methods. Sonochemical degradation was directly proportional to the measured cavitation produced by these sonophotocatalysts. Our work suggests that simple nanostructuring of current sonosensitizers to enable on-site cavitation greatly enhances sonochemical reaction rates.
Keywords: Cavitation nuclei; Pulsed ultrasound; Sonophotocatalysis; Titanium dioxide nanoparticles.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Synthesis of TiO2/WO3 nanoparticles via sonochemical approach for the photocatalytic degradation of methylene blue under visible light illumination.Ultrason Sonochem. 2014 Nov;21(6):1964-8. doi: 10.1016/j.ultsonch.2014.02.015. Epub 2014 Feb 26. Ultrason Sonochem. 2014. PMID: 24629580
-
Ultrasound to improve both synthesis and pollutants degradation based on metal nanoparticles supported on TiO2.Ultrason Sonochem. 2019 Mar;51:462-468. doi: 10.1016/j.ultsonch.2018.07.011. Epub 2018 Jul 6. Ultrason Sonochem. 2019. PMID: 30001881
-
Sonochemical synthesis of Au-TiO2 nanoparticles for the sonophotocatalytic degradation of organic pollutants in aqueous environment.Ultrason Sonochem. 2009 Mar;16(3):316-20. doi: 10.1016/j.ultsonch.2008.10.010. Epub 2008 Oct 22. Ultrason Sonochem. 2009. PMID: 19028129
-
An overview on ZnO-based sonophotocatalytic mitigation of aqueous phase pollutants.Chemosphere. 2023 Aug;333:138873. doi: 10.1016/j.chemosphere.2023.138873. Epub 2023 May 8. Chemosphere. 2023. PMID: 37164195 Review.
-
Application of Photocatalysis and Sonocatalysis for Treatment of Organic Dye Wastewater and the Synergistic Effect of Ultrasound and Light.Molecules. 2023 Apr 25;28(9):3706. doi: 10.3390/molecules28093706. Molecules. 2023. PMID: 37175115 Free PMC article. Review.
Cited by
-
Active and highly durable supported catalysts for proton exchange membrane electrolysers.EES Catal. 2024 Jun 21;2(5):1139-1151. doi: 10.1039/d4ey00026a. eCollection 2024 Sep 5. EES Catal. 2024. PMID: 39246682 Free PMC article.
-
Ultrasonic Activation of Au Nanoclusters/TiO2: Tuning Hydroxyl Radical Production Through Frequency and Nanocluster Size.Molecules. 2025 Jan 24;30(3):541. doi: 10.3390/molecules30030541. Molecules. 2025. PMID: 39942645 Free PMC article.
-
The Synthesis of Europium-Doped Calcium Carbonate by an Eco-Method as Free Radical Generator Under Low-Intensity Ultrasonic Irradiation for Body Sculpture.Front Bioeng Biotechnol. 2021 Nov 19;9:765630. doi: 10.3389/fbioe.2021.765630. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34869278 Free PMC article.
-
Gas-stabilizing nanoparticles for ultrasound imaging and therapy of cancer.Nano Converg. 2021 Dec 1;8(1):39. doi: 10.1186/s40580-021-00287-2. Nano Converg. 2021. PMID: 34851458 Free PMC article. Review.
-
Overview of Therapeutic Ultrasound Applications and Safety Considerations: 2024 Update.J Ultrasound Med. 2025 Mar;44(3):381-433. doi: 10.1002/jum.16611. Epub 2024 Nov 11. J Ultrasound Med. 2025. PMID: 39526313 Free PMC article. Review.
References
-
- Suslick K.S., Price G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999;29(1):295–326.
-
- McKenzie T.G., Karimi F., Ashokkumar M., Qiao G.G. Ultrasound and sonochemistry for radical polymerization: sound synthesis. Chemistry. 2019;25(21):5372–5388. - PubMed
-
- Thomas R.G., Jonnalagadda U.S., Kwan J.J. Biomedical applications for gas-stabilizing solid cavitation agents. Langmuir. 2019;35(31):10106–10115. - PubMed
-
- Wood R.J., Lee J., Bussemaker M.J. A parametric review of sonochemistry: control and augmentation of sonochemical activity in aqueous solutions. Ultrason. Sonochem. 2017;38:351–370. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

