Clinical outcomes of platinum-based chemotherapy in patients with advanced breast cancer: An 11-year single institutional experience
- PMID: 33799232
- PMCID: PMC8044724
- DOI: 10.1016/j.breast.2021.03.002
Clinical outcomes of platinum-based chemotherapy in patients with advanced breast cancer: An 11-year single institutional experience
Abstract
Background/methods: Although the prognosis of metastatic breast cancer (BC) has improved, some patients still develop high burden metastases or visceral crisis (VC) and polychemotherapy is commonly used in these cases. Data reporting the real effectiveness of this strategy are scanty. Therefore, the outcomes of patients with metastatic BC treated with platinum-based chemotherapy (P-ChT) at the Jules Bordet Institute during the period of January 2008 and December 2018 were retrospectively reviewed. The presence of VC was defined according to ABC 4 criteria.
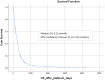
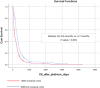
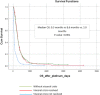
Results: 441 patients were identified: visceral metastases were observed in 430 (97.5%) while 261 (59.2%) presented VC. As for metastatic BC subtype, 255 (57.8%) had ER-positive/HER2-negative, 41 (9.3%) ER-positive/HER2-positive, 34 (7.7%) ER-negative/HER2-positive and 111 (25.1%) triple-negative BC. Median number of prior treatment lines was 3.8 (0-12). Median OS with P-ChT in the entire cohort was 6.13 months. Patients with VC had lower OS than patients without VC (8.6 vs 3.7 months; p < 0.001). On multivariate analysis, the variables correlated with worse OS were hyperbilirubinemia (HR 1.90; 95% CI 1.34-2.75), ECOG ≥2 (HR 1.77; 95% CI 1.13-2.78) and ECOG ≥3 (HR 2.52; 95% CI 1.48-4.28), and >3 previous treatment lines (HR 2.27; 95% CI 1.53-3.21). Of the 261 patients with VC, 106 (40.5%) presented a resolution of the VC which correlated with better OS (9.3 vs 2.0 months, HR 0.27; 95% CI 0.21-0.36).
Conclusion: Patients who overcome VC benefit from P-ChT with OS similar to patients without VC. In this analysis, hyperbilirubinemia, poor ECOG and >3 previous treatment lines were significant prognostic factors in the overall study population.
Keywords: Metastatic breast cancer; Platinum-based chemotherapy; Visceral crisis.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest MAF: none. RSC, SF: Travel grant from Pfizer for the ESMO conference 2019. DE: Funding for his fellowship (2018–2019: Novartis. Speaker fee: Janssen. AA: Advisory role, speaker fees and research funding for his institute from: Roche, Lilly, Amgen, EISAI, BMS, Pfizer, Novartis, MSD, Genomic Health, Ipsen, AstraZeneca, Bayer, Leo Pharma. EA: honoraria and advisory board: Roche/GNE, Novartis, Seattle Genetics and Zodiacs; travel grants: Roche/GNE, GSK/Novartis; co-principal investigator of the LORELEI trial (NCT02273973). Research grant for his institute: Roche/GNE, Astra-Zeneca, Novartis, and Servier.
Figures
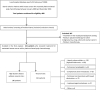
References
-
- Waks A.G., Winer E.P. Breast cancer treatment: a review. J Am Med Assoc. 2019;321:288–300. - PubMed
-
- Harbeck N., Penault-Llorca F., Cortes J. Breast cancer. Nat Rev Dis Primers. 2019;5(1–31) - PubMed
-
- Perou C.M., Sørlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

