Epithelial Membrane Protein 2 Suppresses Non-Small Cell Lung Cancer Cell Growth by Inhibition of MAPK Pathway
- PMID: 33799364
- PMCID: PMC7999101
- DOI: 10.3390/ijms22062944
Epithelial Membrane Protein 2 Suppresses Non-Small Cell Lung Cancer Cell Growth by Inhibition of MAPK Pathway
Abstract
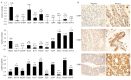
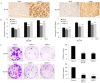
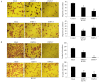
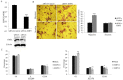
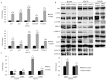
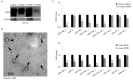
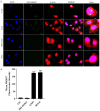
Epithelial membrane proteins (EMP1-3) are involved in epithelial differentiation and carcinogenesis. Dysregulated expression of EMP2 was observed in various cancers, but its role in human lung cancer is not yet clarified. In this study, we analyzed the expression of EMP1-3 and investigated the biological function of EMP2 in non-small cell lung cancer (NSCLC). The results showed that lower expression of EMP1 was significantly correlated with tumor size in primary lung tumors (p = 0.004). Overexpression of EMP2 suppressed tumor cell growth, migration, and invasion, resulting in a G1 cell cycle arrest, with knockdown of EMP2 leading to enhanced cell migration, related to MAPK pathway alterations and disruption of cell cycle regulatory genes. Exosomes isolated from transfected cells were taken up by tumor cells, carrying EMP2-downregulated microRNAs (miRNAs) which participated in regulation of the tumor microenvironment. Our data suggest that decreased EMP1 expression is significantly related to increased tumor size in NSCLC. EMP2 suppresses NSCLC cell growth mainly by inhibiting the MAPK pathway. EMP2 might further affect the tumor microenvironment by regulating tumor microenvironment-associated miRNAs.
Keywords: NSCLC; epithelial membrane protein; exosome; microRNA; tumor pathways.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., et al. WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

