Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study
- PMID: 33799425
- PMCID: PMC8002058
- DOI: 10.3390/ijerph18062977
Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study
Abstract
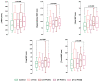
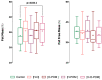
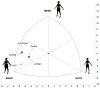
To describe whether polycystic ovary syndrome (PCOS) phenotypes vary in their body composition and skinfold (SKF) thicknesses and if they differ from women without PCOS, a preiminar case-control study was performed. A total of 117 cases were diagnosed using the Rotterdam criteria. Gynecological examinations and transvaginal ultrasound were performed in all women (266 women). Anthropometric measurements including SKF thickness were taken according to the restricted profile protocol of the international standards for the anthropometric evaluation according to the International Society of the Advancement of Kinanthropometry (ISAK). Women with PCOS had higher body mass index and percentage of fat mass with respect to controls. The endomorphy component was also significantly higher in women with PCOS than in controls. Each PCOS phenotype displayed a different representation in the somatochart respect to the others phenotypes and also compared to controls. Women with PCOS had significantly higher ∑7 SKF (p = 0.013), ∑appendicular SKF (p = 0.017) and ∑arm SKF (p = 0.019) than controls. H-O-POM phenotype had higher 7∑ SKF (p = 0.003), ∑appendicular SKF (p = 0.01), ∑arm SKF (0.005), ∑leg SKF, and ∑trunk SKF (0.008) and also a higher fast mass percentage than controls (p = 0.011). In conclusion, body composition evaluated by ISAK protocol is different in women with PCOS, especially in the complete phenotype (H-O-POM). This could have relevant implications in terms of clinical evaluation and follow-up of these women, although more researches in this field are needed.
Keywords: ISAK; anthropometry; polycystic ovary syndrome; somatochart; somatotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lizneva D., Kirubakaran R., Mykhalchenko K., Suturina L., Chernukha G., Diamond M.P., Azziz R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: Systematic review and meta-analysis. Fertil. Steril. 2016;106:1510–1520.e2. doi: 10.1016/j.fertnstert.2016.07.1121. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

