Antioxidant and Signaling Role of Plastid-Derived Isoprenoid Quinones and Chromanols
- PMID: 33799456
- PMCID: PMC7999835
- DOI: 10.3390/ijms22062950
Antioxidant and Signaling Role of Plastid-Derived Isoprenoid Quinones and Chromanols
Abstract
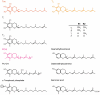
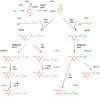
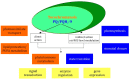
Plant prenyllipids, especially isoprenoid chromanols and quinols, are very efficient low-molecular-weight lipophilic antioxidants, protecting membranes and storage lipids from reactive oxygen species (ROS). ROS are byproducts of aerobic metabolism that can damage cell components, they are also known to play a role in signaling. Plants are particularly prone to oxidative damage because oxygenic photosynthesis results in O2 formation in their green tissues. In addition, the photosynthetic electron transfer chain is an important source of ROS. Therefore, chloroplasts are the main site of ROS generation in plant cells during the light reactions of photosynthesis, and plastidic antioxidants are crucial to prevent oxidative stress, which occurs when plants are exposed to various types of stress factors, both biotic and abiotic. The increase in antioxidant content during stress acclimation is a common phenomenon. In the present review, we describe the mechanisms of ROS (singlet oxygen, superoxide, hydrogen peroxide and hydroxyl radical) production in chloroplasts in general and during exposure to abiotic stress factors, such as high light, low temperature, drought and salinity. We highlight the dual role of their presence: negative (i.e., lipid peroxidation, pigment and protein oxidation) and positive (i.e., contribution in redox-based physiological processes). Then we provide a summary of current knowledge concerning plastidic prenyllipid antioxidants belonging to isoprenoid chromanols and quinols, as well as their structure, occurrence, biosynthesis and function both in ROS detoxification and signaling.
Keywords: antioxidants; lipid peroxidation; oxidative stress; plastochromanol; quinols; reactive oxygen species; signaling pathways; tocochromanols; tocopherols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Edreva A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosyst. Environ. 2005;106:119–133. doi: 10.1016/j.agee.2004.10.022. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

