Modelling Parkinson's Disease: iPSCs towards Better Understanding of Human Pathology
- PMID: 33799491
- PMCID: PMC8000082
- DOI: 10.3390/brainsci11030373
Modelling Parkinson's Disease: iPSCs towards Better Understanding of Human Pathology
Abstract
Parkinson's Disease (PD) is a chronic neurodegenerative disorder characterized by motor and non-motor symptoms, among which are bradykinesia, rigidity, tremor as well as mental symptoms such as dementia. The underlying cause of Parkinson disease is degeneration of dopaminergic neurons. It has been challenging to develop an efficient animal model to accurately represent the complex phenotypes found with PD. However, it has become possible to recapitulate the myriad of phenotypes underlying the PD pathology by using human induced pluripotent stem cell (iPSC) technology. Patient-specific iPSC-derived dopaminergic neurons are available and present an opportunity to study many aspects of the PD phenotypes in a dish. In this review, we report the available data on iPSC-derived neurons derived from PD patients with identified gene mutations. Specifically, we will report on the key phenotypes of the generated iPSC-derived neurons from PD patients with different genetic background. Furthermore, we discuss the relationship these cellular phenotypes have to PD pathology and future challenges and prospects for iPSC modelling and understanding of the pathogenesis of PD.
Keywords: Parkinson’s disease; human pathology; induced pluripotent stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

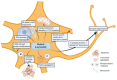
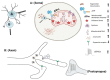
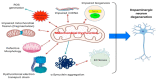
References
-
- Moisan F., Kab S., Mohamed F., Canonico M., Le Guern M., Quintin C., Carcaillon L., Nicolau J., Duport N., Singh-Manoux A., et al. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2016;87:952–957. doi: 10.1136/jnnp-2015-312283. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

