Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes
- PMID: 33799511
- PMCID: PMC7998954
- DOI: 10.3390/ijms22062830
Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes
Abstract
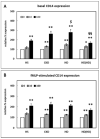
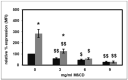
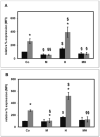
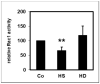
In uremic patients, high-density lipoprotein (HDL) loses its anti-inflammatory features and can even become pro-inflammatory due to an altered protein composition. In chronic kidney disease (CKD), impaired functions of polymorphonuclear leukocytes (PMNLs) contribute to inflammation and an increased risk of cardiovascular disease. This study investigated the effect of HDL from CKD and hemodialysis (HD) patients on the CD14 expression on PMNLs. HDL was isolated using a one-step density gradient centrifugation. Isolation of PMNLs was carried out by discontinuous Ficoll-Hypaque density gradient centrifugation. CD14 surface expression was quantified by flow cytometry. The activity of the small GTPase Rac1 was determined by means of an activation pull-down assay. HDL increased the CD14 surface expression on PMNLs. This effect was more pronounced for HDL isolated from uremic patients. The acute phase protein serum amyloid A (SAA) caused higher CD14 expression, while SAA as part of an HDL particle did not. Lipid raft disruption with methyl-β-cyclodextrin led to a reduced CD14 expression in the absence and presence of HDL. HDL from healthy subjects but not from HD patients decreased the activity of Rac1. Considering the known anti-inflammatory effects of HDL, the finding that even HDL from healthy subjects increased the CD14 expression was unexpected. The pathophysiological relevance of this result needs further investigation.
Keywords: CD14; Rac1; high-density lipoprotein; immunology; inflammation; lipid rafts; polymorphonuclear leukocytes; serum amyloid A.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
High-Density Lipoprotein from Chronic Kidney Disease Patients Modulates Polymorphonuclear Leukocytes.Toxins (Basel). 2019 Feb 1;11(2):73. doi: 10.3390/toxins11020073. Toxins (Basel). 2019. PMID: 30717079 Free PMC article.
-
Serum amyloid A enrichment impairs the anti-inflammatory ability of HDL from diabetic nephropathy patients.J Diabetes Complications. 2017 Oct;31(10):1538-1543. doi: 10.1016/j.jdiacomp.2017.07.005. Epub 2017 Jul 13. J Diabetes Complications. 2017. PMID: 28760652
-
High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A.Cardiovasc Res. 2012 Apr 1;94(1):154-62. doi: 10.1093/cvr/cvs089. Epub 2012 Feb 10. Cardiovasc Res. 2012. PMID: 22328092
-
High-density lipoprotein: structural and functional changes under uremic conditions and the therapeutic consequences.Handb Exp Pharmacol. 2015;224:423-53. doi: 10.1007/978-3-319-09665-0_13. Handb Exp Pharmacol. 2015. PMID: 25522997 Review.
-
Molecular mechanisms underlying uremic toxin-related systemic disorders in chronic kidney disease: focused on β2-microglobulin-related amyloidosis and indoxyl sulfate-induced atherosclerosis-Oshima Award Address 2016.Clin Exp Nephrol. 2019 Feb;23(2):151-157. doi: 10.1007/s10157-018-1588-9. Epub 2018 Jun 5. Clin Exp Nephrol. 2019. PMID: 29869756 Free PMC article. Review.
Cited by
-
Method Matters: Effect of Purification Technology on Neutrophil Phenotype and Function.Front Immunol. 2022 Feb 10;13:820058. doi: 10.3389/fimmu.2022.820058. eCollection 2022. Front Immunol. 2022. PMID: 35222394 Free PMC article.
-
High-Density Lipoproteins in Non-Cardiovascular Diseases.Int J Mol Sci. 2022 Aug 20;23(16):9413. doi: 10.3390/ijms23169413. Int J Mol Sci. 2022. PMID: 36012681 Free PMC article.
References
-
- Rosenson R.S., Brewer H.B., Jr., Davidson W.S., Fayad Z.A., Fuster V., Goldstein J., Hellerstein M., Jiang X.C., Phillips M.C., Rader D.J., et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

