A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®
- PMID: 33799595
- PMCID: PMC7998955
- DOI: 10.3390/mi12030296
A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®
Abstract
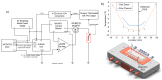
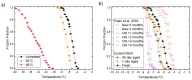
Measurement of ice nucleation (IN) temperature of liquid solutions at sub-ambient temperatures has applications in atmospheric, water quality, food storage, protein crystallography and pharmaceutical sciences. Here we present details on the construction of a temperature-controlled microfluidic platform with multiple individually addressable temperature zones and on-chip temperature sensors for high-throughput IN studies in droplets. We developed, for the first time, automated droplet freezing detection methods in a microfluidic device, using a deep neural network (DNN) and a polarized optical method based on intensity thresholding to classify droplets without manual counting. This platform has potential applications in continuous monitoring of liquid samples consisting of aerosols to quantify their IN behavior, or in checking for contaminants in pure water. A case study of the two detection methods was performed using Snomax® (Snomax International, Englewood, CO, USA), an ideal ice nucleating particle (INP). Effects of aging and heat treatment of Snomax® were studied with Fourier transform infrared (FTIR) spectroscopy and a microfluidic platform to correlate secondary structure change of the IN protein in Snomax® to IN temperature. It was found that aging at room temperature had a mild impact on the ice nucleation ability but heat treatment at 95 °C had a more pronounced effect by reducing the ice nucleation onset temperature by more than 7 °C and flattening the overall frozen fraction curve. Results also demonstrated that our setup can generate droplets at a rate of about 1500/min and requires minimal human intervention for DNN classification.
Keywords: Snomax®; automated detection; deep neural network; high-throughput; ice nucleating particle; machine learning; microfluidic device; polarized light.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- James C., Purnell G., James S.J. A Review of Novel and Innovative Food Freezing Technologies. Food Bioprocess Technol. 2015;8:1616–1634. doi: 10.1007/s11947-015-1542-8. - DOI
-
- Kiani H., Sun D.-W. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011;22:407–426. doi: 10.1016/j.tifs.2011.04.011. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

