Measuring Pre- and Post-Copulatory Sexual Selection and Their Interaction in Socially Monogamous Species with Extra-Pair Paternity
- PMID: 33799610
- PMCID: PMC7999480
- DOI: 10.3390/cells10030620
Measuring Pre- and Post-Copulatory Sexual Selection and Their Interaction in Socially Monogamous Species with Extra-Pair Paternity
Abstract
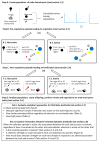
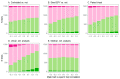
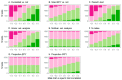
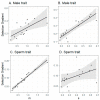
When females copulate with multiple males, pre- and post-copulatory sexual selection may interact synergistically or in opposition. Studying this interaction in wild populations is complex and potentially biased, because copulation and fertilization success are often inferred from offspring parentage rather than being directly measured. Here, I simulated 15 species of socially monogamous birds with varying levels of extra-pair paternity, where I could independently cause a male secondary sexual trait to improve copulation success, and a sperm trait to improve fertilization success. By varying the degree of correlation between the male and sperm traits, I show that several common statistical approaches, including univariate selection gradients and paired t-tests comparing extra-pair males to the within-pair males they cuckolded, can give highly biased results for sperm traits. These tests should therefore be avoided for sperm traits in socially monogamous species with extra-pair paternity, unless the sperm trait is known to be uncorrelated with male trait(s) impacting copulation success. In contrast, multivariate selection analysis and a regression of the proportion of extra-pair brood(s) sired on the sperm trait of the extra-pair male (including only broods where the male sired ≥1 extra-pair offspring) were unbiased, and appear likely to be unbiased under a broad range of conditions for this mating system. In addition, I investigated whether the occurrence of pre-copulatory selection impacted the strength of post-copulatory selection, and vice versa. I found no evidence of an interaction under the conditions simulated, where the male trait impacted only copulation success and the sperm trait impacted only fertilization success. Instead, direct selection on each trait was independent of whether the other trait was under selection. Although pre- and post-copulatory selection strength was independent, selection on the two traits was positively correlated across species because selection on both traits increased with the frequency of extra-pair copulations in these socially monogamous species.
Keywords: cryptic female choice; evolution; extra-pair paternity; fertilization; gametes; mating systems; passerines; post-copulatory sexual selection; pre-copulatory sexual selection; reproduction; sperm competition.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Darwin C. The Descent of Man, and Selection in Relation to Sex. John Murray; London, UK: 1871.
-
- Andersson M. Sexual Selection. Princeton University Press; Princeton, NJ, USA: 1994.
-
- Parker G. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x. - DOI
-
- Eberhard W. Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press; Princeton, NJ, USA: 1996.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

