Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis
- PMID: 33799634
- PMCID: PMC7999402
- DOI: 10.3390/molecules26061531
Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis
Abstract
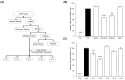
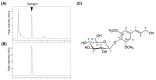
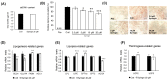
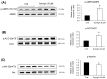
Cirsium brevicaule A. GRAY is a wild perennial herb, and its roots (CbR) have traditionally been used as both food and medicine on the Japanese islands of Okinawa and Amami. The present study evaluated the antiadipogenic effect of CbR using mouse embryonic fibroblast cell line 3T3-L1 from JCRB cell bank. Dried CbR powder was serially extracted with solvents of various polarities, and these crude extracts were tested for antiadipogenic activity. Treatment with the methanol extract of CbR showed a significant suppression of lipid accumulation in 3T3-L1 cells. Methanol extract of CbR was then fractionated and subjected to further activity analyses. The phenylpropanoid glycosidic molecule syringin was identified as an active compound. Syringin dose dependently suppressed lipid accumulation of 3T3-L1 cells without cytotoxicity, and significantly reduced the expressions of peroxisome proliferator-activated receptor gamma, the master regulator of adipogenesis, and other differentiation markers. It was demonstrated that syringin effectively enhanced the phosphorylation of the AMP-activated protein kinase and acetyl-CoA carboxylase. These results indicate that syringin attenuates adipocyte differentiation, adipogenesis, and promotes lipid metabolism; thus, syringin may potentially serve as a therapeutic candidate for treatment of obesity.
Keywords: 3T3-L1 cells; Cirsium brevicaule A. GRAY; anti-adipogenesis; syringin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Standardized Cirsium setidens Nakai Ethanolic Extract Suppresses Adipogenesis and Regulates Lipid Metabolisms in 3T3-L1 Adipocytes and C57BL/6J Mice Fed High-Fat Diets.J Med Food. 2017 Aug;20(8):763-776. doi: 10.1089/jmf.2017.3965. Epub 2017 Jul 7. J Med Food. 2017. PMID: 28686516
-
Korean Chungtaejeon tea extract attenuates body weight gain in C57BL/6J-Lep ob/ob mice and regulates adipogenesis and lipolysis in 3T3-L1 adipocytes.J Integr Med. 2017 Jan;15(1):56-63. doi: 10.1016/S2095-4964(17)60321-2. J Integr Med. 2017. PMID: 28088260
-
Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes.Int J Mol Sci. 2021 Oct 22;22(21):11409. doi: 10.3390/ijms222111409. Int J Mol Sci. 2021. PMID: 34768840 Free PMC article.
-
New Perspectives on the Molecular Action of Metformin in the Context of Cellular Transduction and Adipogenesis.Int J Mol Sci. 2025 Apr 14;26(8):3690. doi: 10.3390/ijms26083690. Int J Mol Sci. 2025. PMID: 40332335 Free PMC article. Review.
-
Syringin: Plant Source, Traditional Uses, Anti-Cancer, Brain Protection, and Related Pharmacological Properties.Chem Biodivers. 2025 Apr;22(4):e202402272. doi: 10.1002/cbdv.202402272. Epub 2024 Dec 26. Chem Biodivers. 2025. PMID: 39552511 Review.
Cited by
-
Syringin: a naturally occurring compound with medicinal properties.Front Pharmacol. 2024 Jul 22;15:1435524. doi: 10.3389/fphar.2024.1435524. eCollection 2024. Front Pharmacol. 2024. PMID: 39104400 Free PMC article. Review.
-
Identification of New Polyacetylenes from Dendropanax morbifera with PPAR-α Activity Study.Molecules. 2024 Dec 16;29(24):5942. doi: 10.3390/molecules29245942. Molecules. 2024. PMID: 39770031 Free PMC article.
-
Origanum majorana L.: A Nutritional Supplement With Immunomodulatory Effects.Front Nutr. 2021 Sep 22;8:748031. doi: 10.3389/fnut.2021.748031. eCollection 2021. Front Nutr. 2021. PMID: 34631774 Free PMC article.
-
Hemp Seed-Based Foods and Processing By-Products Are Sustainable Rich Sources of Nutrients and Plant Metabolites Supporting Dietary Biodiversity, Health, and Nutritional Needs.Foods. 2025 Mar 4;14(5):875. doi: 10.3390/foods14050875. Foods. 2025. PMID: 40077578 Free PMC article.
-
Isolation, Characterization and In Silico Studies of Secondary Metabolites from the Whole Plant of Polygala inexpectata Peşmen & Erik.Molecules. 2022 Jan 21;27(3):684. doi: 10.3390/molecules27030684. Molecules. 2022. PMID: 35163950 Free PMC article.
References
-
- World Health Organization Obesity and Overweight. [(accessed on 17 February 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#....
-
- World Health Organization Noncommunicable Diseases Progress Monitor. WHO. [(accessed on 20 November 2020)];2017 Available online: https://ncdalliance.org/resources/who-ncds-progress-monitor-2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

