A Tryptophan 'Gate' in the CRISPR-Cas3 Nuclease Controls ssDNA Entry into the Nuclease Site, That When Removed Results in Nuclease Hyperactivity
- PMID: 33799639
- PMCID: PMC8001533
- DOI: 10.3390/ijms22062848
A Tryptophan 'Gate' in the CRISPR-Cas3 Nuclease Controls ssDNA Entry into the Nuclease Site, That When Removed Results in Nuclease Hyperactivity
Abstract
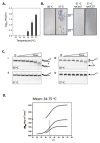
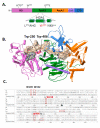
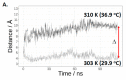
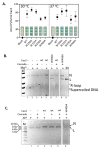
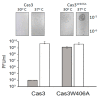
Cas3 is a ssDNA-targeting nuclease-helicase essential for class 1 prokaryotic CRISPR immunity systems, which has been utilized for genome editing in human cells. Cas3-DNA crystal structures show that ssDNA follows a pathway from helicase domains into a HD-nuclease active site, requiring protein conformational flexibility during DNA translocation. In genetic studies, we had noted that the efficacy of Cas3 in CRISPR immunity was drastically reduced when temperature was increased from 30 °C to 37 °C, caused by an unknown mechanism. Here, using E. coli Cas3 proteins, we show that reduced nuclease activity at higher temperature corresponds with measurable changes in protein structure. This effect of temperature on Cas3 was alleviated by changing a single highly conserved tryptophan residue (Trp-406) into an alanine. This Cas3W406A protein is a hyperactive nuclease that functions independently from temperature and from the interference effector module Cascade. Trp-406 is situated at the interface of Cas3 HD and RecA1 domains that is important for maneuvering DNA into the nuclease active site. Molecular dynamics simulations based on the experimental data showed temperature-induced changes in positioning of Trp-406 that either blocked or cleared the ssDNA pathway. We propose that Trp-406 forms a 'gate' for controlling Cas3 nuclease activity via access of ssDNA to the nuclease active site. The effect of temperature in these experiments may indicate allosteric control of Cas3 nuclease activity caused by changes in protein conformations. The hyperactive Cas3W406A protein may offer improved Cas3-based genetic editing in human cells.
Keywords: CRISPR; Cas3; genome editing; helicase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Makarova K.S., Wolf Y.I., Iranzo J., Shmakov S.A., Alkhnbashi O.S., Brouns S.J.J., Charpentier E., Cheng D., Haft D.H., Horvath P., et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. MicroBiol. 2020;18:67–83. doi: 10.1038/s41579-019-0299-x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

