Post-Transplant Cyclophosphamide and Tacrolimus-Mycophenolate Mofetil Combination Governs GVHD and Immunosuppression Need, Reducing Late Toxicities in Allogeneic Peripheral Blood Hematopoietic Cell Transplantation from HLA-Matched Donors
- PMID: 33799685
- PMCID: PMC7998305
- DOI: 10.3390/jcm10061173
Post-Transplant Cyclophosphamide and Tacrolimus-Mycophenolate Mofetil Combination Governs GVHD and Immunosuppression Need, Reducing Late Toxicities in Allogeneic Peripheral Blood Hematopoietic Cell Transplantation from HLA-Matched Donors
Abstract
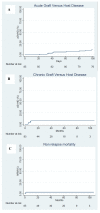
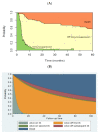
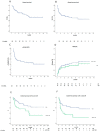
Combined direct antineoplastic activity and the long-lasting immunological effects of allogeneic hematopoietic cell transplant (HCT) can cure many hematological malignancies, but broad adoption requires non-relapse mortality (NRM) rates and graft-versus-host disease (GVHD) control. Recently, posttransplant cyclophosphamide (PTCy) given after a bone marrow transplant significantly reduced GVHD-incidence, while PTCy given with tacrolimus/mofetil mycophenolate (T/MMF) showed activity following allogeneic peripheral blood stem cell transplantation (alloPBSCT). Here, we report the experience of a larger cohort (85 consecutive patients) and expanded follow-up period (03/2011-12/2019) with high-risk hematological malignancies who received alloPBSCT from Human-Leukocyte-Antigens HLA-matched unrelated/related donors. GVHD-prophylaxis was PTCy 50 mg/kg (days+3 and +4) combined with T/MMF (day+5 forward). All patients stopped MMF on day+28 with day+110 = median tacrolimus discontinuation. Cumulative incidences were 12% for acute and 7% for chronic GVHD- and no GVHD-attributed deaths. For surviving patients, the 12, 24, and 36-month probabilities of being off immunosuppression were 92, 96, and 96%, respectively. After a 36-month median follow-up, NRM was 4%; median event-free survival (EFS) and overall survival (OS) had yet to occur. One- and two-year chronic GVHD-EFS results were 57% (95% CI, 46-68%) and 53% (95% CI, 45-61%), respectively, with limited late infections and long-term organ toxicities. Disease relapse caused the most treatment failures (38% at 2 years), but low transplant toxicity allowed many patients (14/37, 38%) to receive donor lymphocyte infusions as a post-relapse strategy. We confirmed that PTCy+T/MMF treatment effectively prevented acute and chronic GVHD and limited NRM to unprecedented low rates without loss of disease control efficacy in an expanded patient cohort. This trial is registered at U.S. National Library of Medicine as #NCT02300571.
Keywords: allogeneic hematopoietic cell transplantation; graft-versus-host disease; immunosuppression modulation; long term outcomes; post-transplant cyclophosphamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Post-Transplant Cyclophosphamide and Tacrolimus-Mycophenolate Mofetil Combination Prevents Graft-versus-Host Disease in Allogeneic Peripheral Blood Hematopoietic Cell Transplantation from HLA-Matched Donors.Biol Blood Marrow Transplant. 2017 Mar;23(3):459-466. doi: 10.1016/j.bbmt.2016.12.636. Epub 2016 Dec 27. Biol Blood Marrow Transplant. 2017. PMID: 28039079 Clinical Trial.
-
Graft-versus-Host Disease Prophylaxis in Unrelated Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil.Biol Blood Marrow Transplant. 2016 Jun;22(6):1037-1042. doi: 10.1016/j.bbmt.2016.03.004. Epub 2016 Mar 10. Biol Blood Marrow Transplant. 2016. PMID: 26970381 Clinical Trial.
-
A phase II study of post-transplant cyclophosphamide combined with tacrolimus for GVHD prophylaxis after HLA-matched related/unrelated allogeneic hematopoietic stem cell transplantation.Int J Hematol. 2022 Jan;115(1):77-86. doi: 10.1007/s12185-021-03228-1. Epub 2021 Sep 29. Int J Hematol. 2022. PMID: 34586587 Clinical Trial.
-
Successful Outcome in Patients with Myelofibrosis Undergoing Allogeneic Donor Hematopoietic Cell Transplantation Using Reduced Doses of Post-Transplantation Cyclophosphamide: Challenges and Review of the Literature.Transplant Cell Ther. 2023 Jul;29(7):473.e1-473.e6. doi: 10.1016/j.jtct.2023.04.008. Epub 2023 Apr 20. Transplant Cell Ther. 2023. PMID: 37086849 Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
Cited by
-
Optimizing Outcomes in Mismatched Unrelated Donor Allogeneic Transplantation: Post-Transplant Cyclophosphamide's Dual Impact on Graft versus Host Disease Incidence and Overall Survival: Retrospective Analysis on Behalf of Polish Adult Leukemia Group.J Clin Med. 2024 Jun 18;13(12):3569. doi: 10.3390/jcm13123569. J Clin Med. 2024. PMID: 38930096 Free PMC article.
-
Risk factors for graft-versus-host-disease after donor lymphocyte infusion following T-cell depleted allogeneic stem cell transplantation.Front Immunol. 2024 Mar 13;15:1335341. doi: 10.3389/fimmu.2024.1335341. eCollection 2024. Front Immunol. 2024. PMID: 38545096 Free PMC article.
-
Posttransplant cyclophosphamide beyond haploidentical transplantation.Ann Hematol. 2024 May;103(5):1483-1491. doi: 10.1007/s00277-023-05300-8. Epub 2023 Jun 1. Ann Hematol. 2024. PMID: 37261557 Review.
-
Comparison of Cyclophosphamide-Based Graft Versus Host Disease Prophylaxis after "Allogeneic Stem Cell Transplantation from 9/10 HLA Matched Unrelated Donor'' with Standard Graft Versus Host Disease Prophylaxis after "10/10 HLA Matched Relative Donor'' Transplant.Int J Hematol Oncol Stem Cell Res. 2024 Jul 1;18(3):227-239. doi: 10.18502/ijhoscr.v18i3.16103. Int J Hematol Oncol Stem Cell Res. 2024. PMID: 39257713 Free PMC article.
-
Nonmyeloablative, HLA-Mismatched Unrelated Peripheral Blood Transplantation with High-Dose Post-Transplantation Cyclophosphamide.Transplant Cell Ther. 2021 Nov;27(11):909.e1-909.e6. doi: 10.1016/j.jtct.2021.08.013. Epub 2021 Aug 20. Transplant Cell Ther. 2021. PMID: 34425261 Free PMC article. Clinical Trial.
References
-
- Storb R., Gyurkocza B., Storer B.E., Sorror M.L., Blume K., Niederwieser D., Chauncey T.R., Pulsipher M.A., Petersen F.B., Sahebi F., et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2013;31:1530–1538. doi: 10.1200/JCO.2012.45.0247. - DOI - PMC - PubMed
-
- McDonald G.B., Sandmaier B.M., Mielcarek M., Sorror M., Pergam S.A., Cheng G.S., Hingorani S., Boeckh M., Flowers M.D., Lee S.J., et al. Survival, Nonrelapse Mortality, and Relapse-Related Mortality After Allogeneic Hematopoietic Cell Transplantation: Comparing 2003–2007 Versus 2013–2017 Cohorts. Ann. Intern. Med. 2020;172:229–239. doi: 10.7326/M19-2936. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

