Chromatin-Spliceosome Mutations in Acute Myeloid Leukemia
- PMID: 33799787
- PMCID: PMC7999050
- DOI: 10.3390/cancers13061232
Chromatin-Spliceosome Mutations in Acute Myeloid Leukemia
Abstract
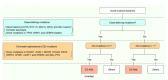
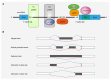
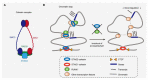
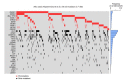
Recent genetic studies on large patient cohorts with acute myeloid leukemia (AML) have cataloged a comprehensive list of driver mutations, resulting in the classification of AML into distinct genomic subgroups. Among these subgroups, chromatin-spliceosome (CS)-AML is characterized by mutations in the spliceosome, cohesin complex, transcription factors, and chromatin modifiers. Class-defining mutations of CS-AML are also frequently identified in myelodysplastic syndrome (MDS) and secondary AML, indicating the molecular similarity among these diseases. CS-AML is associated with myelodysplasia-related changes in hematopoietic cells and poor prognosis, and, thus, can be treated using novel therapeutic strategies and allogeneic stem cell transplantation. Functional studies of CS-mutations in mice have revealed that CS-mutations typically cause MDS-like phenotypes by altering the epigenetic regulation of target genes. Moreover, multiple CS-mutations often synergistically induce more severe phenotypes, such as the development of lethal MDS/AML, suggesting that the accumulation of many CS-mutations plays a crucial role in the progression of MDS/AML. Indeed, the presence of multiple CS-mutations is a stronger indicator of CS-AML than a single mutation. This review summarizes the current understanding of the genetic and clinical features of CS-AML and the functional roles of driver mutations characterizing this unique category of AML.
Keywords: acute myeloid leukemia; chromatin; cohesin; epigenetic regulation; hematopoiesis; myelodysplastic syndrome; splicing factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

