Fucoidan But Not 2'-Fucosyllactose Inhibits Human Norovirus Replication in Zebrafish Larvae
- PMID: 33799811
- PMCID: PMC8001738
- DOI: 10.3390/v13030461
Fucoidan But Not 2'-Fucosyllactose Inhibits Human Norovirus Replication in Zebrafish Larvae
Abstract
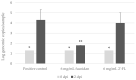
Human noroviruses (hNoVs) cause heavy disease burden worldwide and there is no clinically approved vaccination or antiviral hitherto. In this study, with the use of a zebrafish larva in vivo platform, we investigated the anti-hNoV potentials of fucoidan (from brown algae Fucus vesiculosus) and 2'-Fucosyllactose (2'-FL). As a result, although both fucoidan and 2'-FL were able to block hNoV GII.4 virus-like particle (VLPs) from binding to type A saliva as expected, only fucoidan, but not 2'-FL, was able to inhibit the replication of hNoV GII.P16-GII.4 in zebrafish larvae, indicating the possible needs of higher molecular weights for fucosylated carbohydrates to exert anti-hNoV effect.
Keywords: 2′-fucosyllactose; antiviral; fucoidan; norovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
2'-Fucosyllactose inhibits human norovirus replication in human intestinal enteroids.J Virol. 2025 Feb 25;99(2):e0093824. doi: 10.1128/jvi.00938-24. Epub 2025 Jan 10. J Virol. 2025. PMID: 39791912 Free PMC article.
-
Use of Zebrafish Embryos To Reproduce Human Norovirus and To Evaluate Human Norovirus Infectivity Decay after UV Treatment.Appl Environ Microbiol. 2023 Apr 26;89(4):e0011523. doi: 10.1128/aem.00115-23. Epub 2023 Mar 21. Appl Environ Microbiol. 2023. PMID: 36943055 Free PMC article.
-
A robust human norovirus replication model in zebrafish larvae.PLoS Pathog. 2019 Sep 19;15(9):e1008009. doi: 10.1371/journal.ppat.1008009. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31536612 Free PMC article.
-
Interaction of microorganisms within leafy green phyllospheres: Where do human noroviruses fit in?Int J Food Microbiol. 2017 Oct 3;258:28-37. doi: 10.1016/j.ijfoodmicro.2017.07.010. Epub 2017 Jul 25. Int J Food Microbiol. 2017. PMID: 28755583 Review.
-
Norovirus gastroenteritis, increased understanding and future antiviral options.Curr Opin Investig Drugs. 2008 Feb;9(2):146-51. Curr Opin Investig Drugs. 2008. PMID: 18246517 Review.
Cited by
-
Experimental Methods to Study the Pathogenesis of Human Enteric RNA Viruses.Viruses. 2021 May 25;13(6):975. doi: 10.3390/v13060975. Viruses. 2021. PMID: 34070283 Free PMC article. Review.
-
2'-Fucosyllactose Inhibits Human Norovirus Replication in Human Intestinal Enteroids.bioRxiv [Preprint]. 2024 May 30:2024.05.30.596597. doi: 10.1101/2024.05.30.596597. bioRxiv. 2024. Update in: J Virol. 2025 Feb 25;99(2):e0093824. doi: 10.1128/jvi.00938-24. PMID: 38853945 Free PMC article. Updated. Preprint.
-
Zebrafish as a model organism for virus disease research: Current status and future directions.Heliyon. 2024 Jul 2;10(13):e33865. doi: 10.1016/j.heliyon.2024.e33865. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39071624 Free PMC article.
-
Fucoidan and Derived Oligo-Fucoses: Structural Features with Relevance in Competitive Inhibition of Gastrointestinal Norovirus Binding.Mar Drugs. 2021 Oct 21;19(11):591. doi: 10.3390/md19110591. Mar Drugs. 2021. PMID: 34822462 Free PMC article.
-
Inhibition of Norovirus GII.4 binding to HBGAs by Sargassum fusiforme polysaccharide.Biosci Rep. 2024 Sep 25;44(9):BSR20240092. doi: 10.1042/BSR20240092. Biosci Rep. 2024. PMID: 39158037 Free PMC article.
References
-
- WHO (World Health Organization) WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. WHO; Geneva, Switzerland: 2015. [(accessed on 1 January 2021)]. Available online: https://apps.who.int/iris/handle/10665/199350.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

